Preparation method of grain size controllable ordered mesoporous Ni nanoparticles
A nanoparticle and mesoporous technology, which is applied in the field of preparation of ordered mesoporous Ni nanoparticles, can solve problems such as irregular shapes, and achieve the effects of controllable particle size, easy control of preparation parameters, and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation method of ordered mesoporous Ni nanoparticles with controllable particle size, the steps are as follows:
[0021] 1) First configure a certain amount of pH=2 hydrochloric acid solution, and then add a certain amount of nickel chloride hexahydrate (NiCl 2 ·6H 2 O), palladium chloride (PdCl) 2 ), hydrochloric acid solution and nonionic surfactant cetyl ethoxylate (Brij 58) were heated and stirred continuously at 80°C until a homogeneous solute liquid crystal mixture was formed. where NiCl 2 ·6H 2 O: PdCl 2 : Aqueous HCl: The mass ratio of Brij 58 is 3:1.25×10 -2 :2.75:5.
[0022] 2) Add dimethylaminoborane (DMAB) to the solute liquid crystal mixture prepared in step 1) to jointly reduce Pd ions and Ni ions (wherein dimethylaminoborane and NiCl) 2 ·6H 2 The mass ratio of O was (1.0-1.3):3, and the reduction reaction temperature was 15°C. At the beginning of the reduction reaction, due to the standard electrode potential of Pd (E 0 =0.915V) higher ...
Embodiment 2
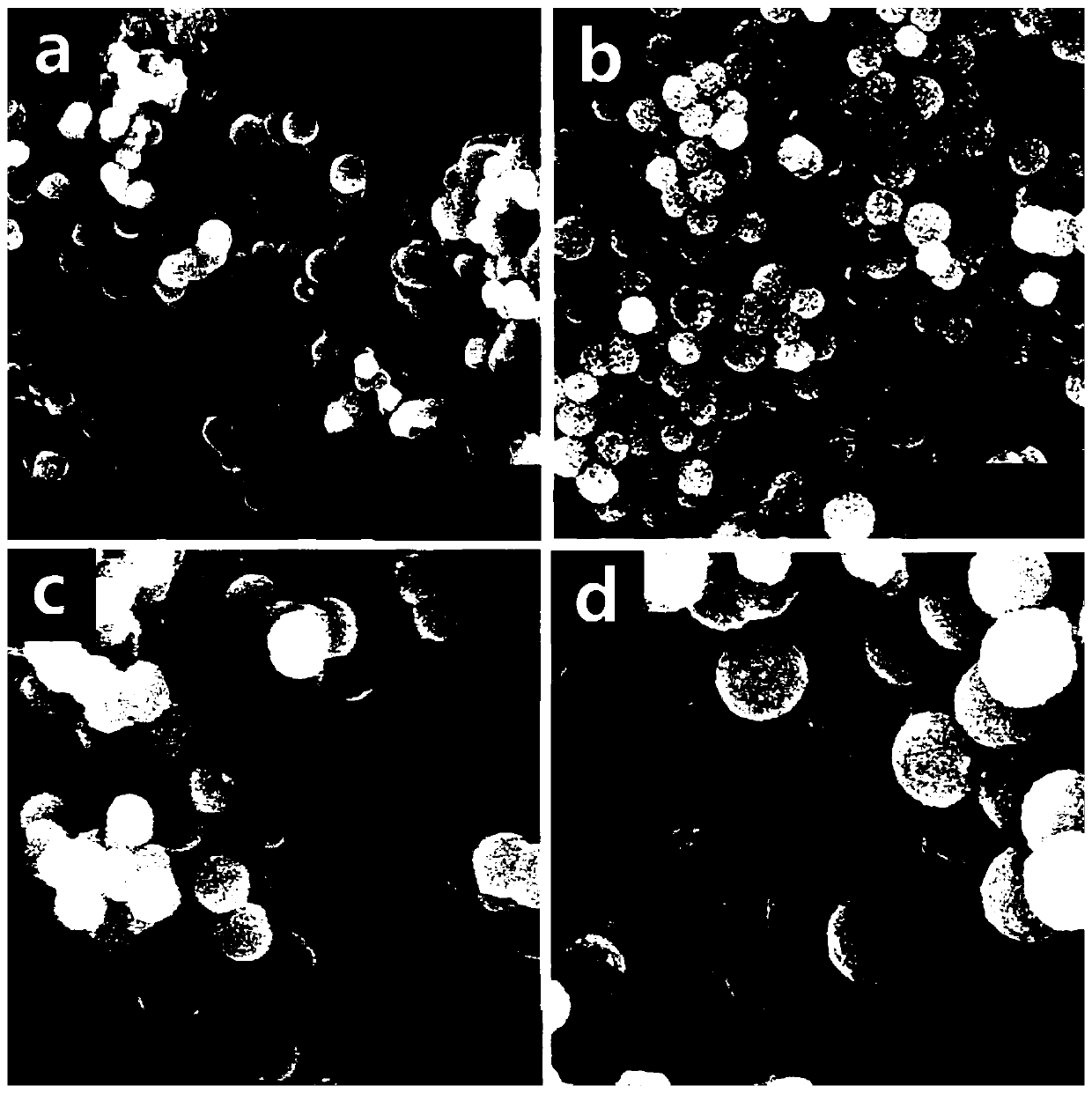
[0026] The preparation method of ordered mesoporous Ni nanoparticles with controllable particle size, the steps are the same as those in Example 1, the difference is that the reduction reaction temperature in step 2) is 20°C.
[0027] The mesoporous order of the ordered mesoporous Ni nanoparticles with controlled particle size prepared by this method is partially destroyed.
Embodiment 3
[0028] Example 3 (ie comparative example)
[0029] The preparation method of ordered mesoporous Ni nanoparticles with controllable particle size, the steps are the same as those in Example 1, the difference is that the reduction reaction temperature in step 2) is 30°C.
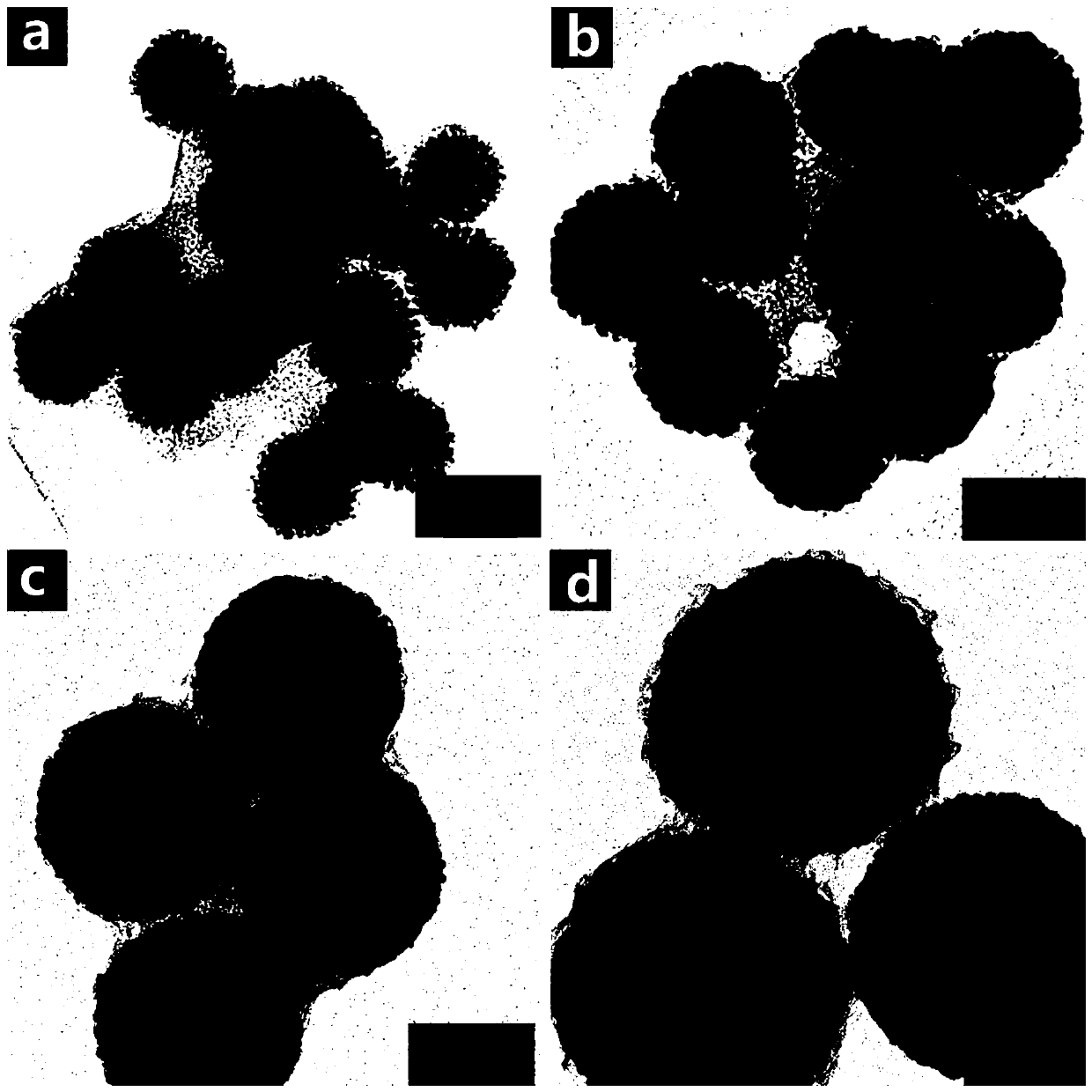
[0030] The mesoporous order of the ordered mesoporous Ni nanoparticles with controlled particle size prepared by this method is completely destroyed, as image 3 shown. The experimental results show that the higher reaction temperature will increase the reaction rate, thereby destroying the order of the mesopores.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com