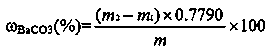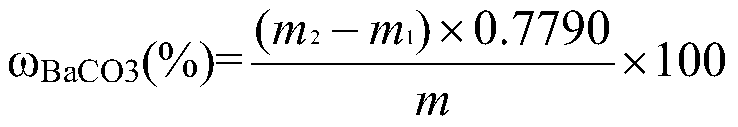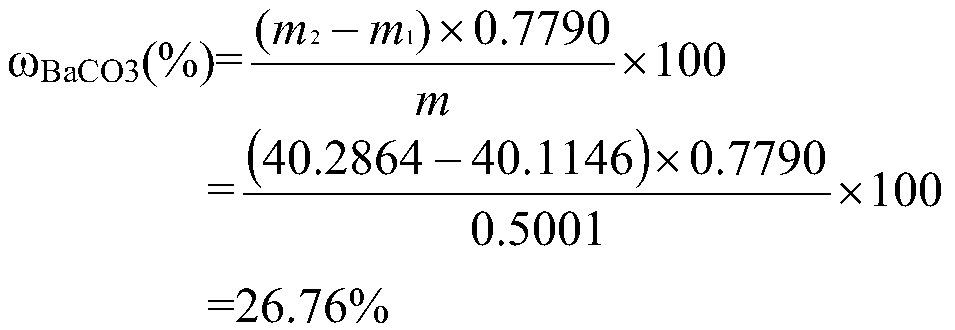Method for detecting content of barium carbonate in witherite
A detection method, witherite technology, applied in the field of chemical detection, can solve the problems of high analysis results, separation of barium sulfate and silicate, etc., and achieve the effect of large detection range, simple and easy-to-learn detection method, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Determination of barium carbonate content in witherite 1# sample
[0038] A. Sample preparation: crush and grind witherite 1# to a particle size of ≤0.125mm, and dry in an oven at a constant temperature of 135°C±5°C for 1.5h;
[0039] B. Prepare the following reagents and solutions: 1+1 hydrochloric acid solution, potassium dichromate solution with a concentration of 20g / L, acetic acid-ammonium acetate buffer solution with pH=4~5, 0.1% methyl red indicator, concentration 100g / L L sodium hydroxide solution, silver nitrate solution with a concentration of 17g / L; the reagents and water used, unless otherwise specified, refer to analytical reagents and third-grade water not lower than those specified in GB / 6682; test The reagents and solutions used in the test are all prepared according to the provisions of GB / T603-2002 "Preparation of Preparations and Products Used in Chemical Reagent Test Methods";
[0040] C. Determination:
[0041] 1) Sample dissolution: Weigh 0.5001g...
Embodiment 2
[0054] Determination of barium carbonate content in witherite 2# sample
[0055] A. Sample preparation: crush and grind witherite 2# to a particle size of ≤0.125mm, and dry in an oven at a constant temperature of 135°C±5°C for 1.8h;
[0056] B. Prepare the following reagents and solutions: 1+1 hydrochloric acid solution, potassium dichromate solution with a concentration of 20g / L, acetic acid-ammonium acetate buffer solution with pH=4~5, 0.1% methyl red indicator, concentration 100g / L L sodium hydroxide solution, silver nitrate solution with a concentration of 17g / L; the reagents and water used, unless otherwise specified, refer to analytical reagents and third-grade water not lower than those specified in GB / 6682; test The reagents and solutions used in the test are all prepared according to the provisions of GB / T603-2002 "Preparation of Preparations and Products Used in Chemical Reagent Test Methods";
[0057] C. Determination:
[0058] 1) Sample dissolution: Weigh 0.5000g...
Embodiment 3
[0071] Determination of barium carbonate content in witherite 3# sample
[0072] A. Sample preparation: crush and grind witherite 3# to a particle size of ≤0.125mm, and dry in an oven at a constant temperature of 135°C±5°C for 2 hours;
[0073] B. Prepare the following reagents and solutions: 1+1 hydrochloric acid solution, potassium dichromate solution with a concentration of 20g / L, acetic acid-ammonium acetate buffer solution with pH=4~5, 0.1% methyl red indicator, concentration 100g / L L sodium hydroxide solution, silver nitrate solution with a concentration of 17g / L; the reagents and water used, unless otherwise specified, refer to analytical reagents and third-grade water not lower than those specified in GB / 6682; test The reagents and solutions used in the test are all prepared according to the provisions of GB / T603-2002 "Preparation of Preparations and Products Used in Chemical Reagent Test Methods";
[0074] C. Determination:
[0075] 1) Sample dissolution: Weigh 0.50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com