Amino terminal brain natriuretic peptide precursor polypeptide, antibody, preparation method thereof, detection kit and detection method thereof
A detection kit and amino terminal technology, applied in the biological field, can solve the problems of inaccurate detection results, poor affinity, sensitive glycosylation sites, etc., to increase immunogenicity, improve detection sensitivity and accuracy of detection results, The effect of improving affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] The embodiment of the present invention also provides a preparation method of the antibody, comprising:
[0057] S110, injecting the coupling protein into the subcutaneous tissue of the mouse to obtain the immunized mouse;
[0058] S120, fusing the myeloma cells with the spleen cells of the immunized mouse to obtain hybridoma cells;
[0059] S130, screening positive hybridoma cells capable of producing NT-proBNP antibody; and
[0060] S140, injecting the positive hybridoma cells into the peritoneal cavity of other mice for culture, and obtaining the amino-terminal BNP precursor antibody in the ascites of the cultured mice.
[0061] An embodiment of the present invention also provides a detection kit for N-terminal N-terminal natriuretic peptide precursor, comprising: a solid phase carrier coated with a first antibody and a second antibody labeled with a chemiluminescence molecule, and the first antibody and the second antibody Secondary antibodies are different.
[0...
Embodiment
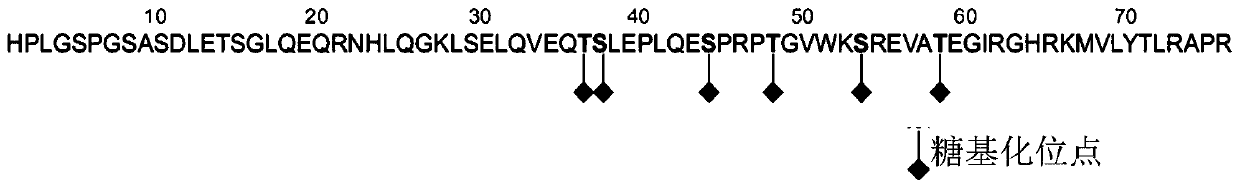
[0084] (1) Immunogen preparation (amino-terminal BNP precursor polypeptide synthesis):
[0085] The following three polypeptides were synthesized by Shanghai Jill Biochemical Biochemistry. The first polypeptide SEQ ID NO.6, the second polypeptide SEQ ID NO.7 and the third polypeptide SEQ ID NO.8 were respectively coupled to the carrier protein BSA to obtain the first Coupling protein, secondary coupling protein and tertiary coupling protein.
[0086] (2) Preparation of N-terminal BNP precursor monoclonal antibody:
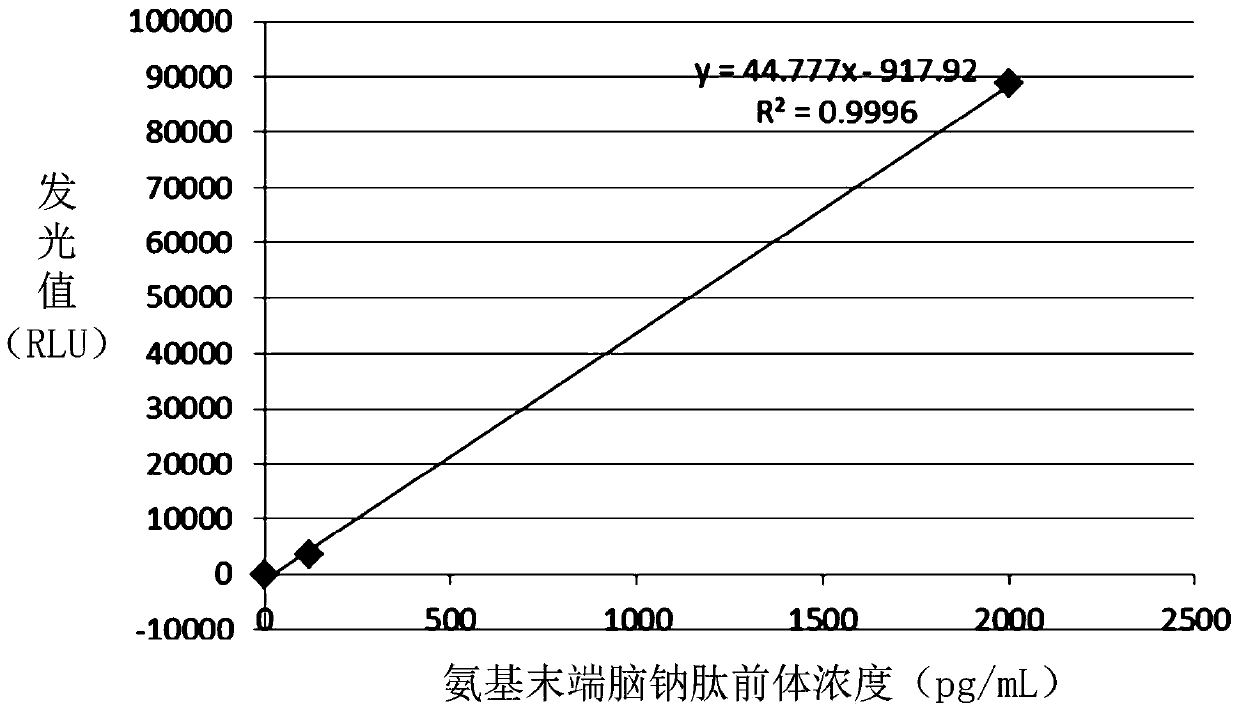
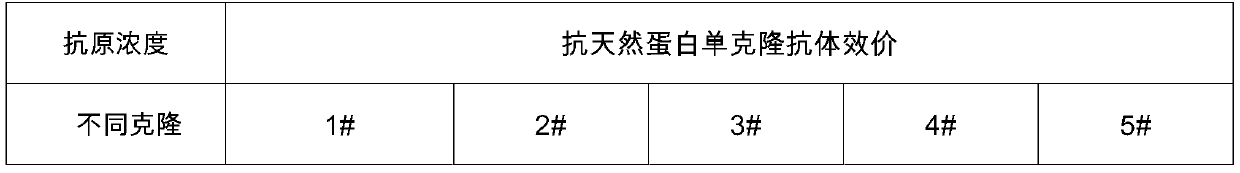
[0087] The first coupling protein, the second coupling protein and the third coupling protein obtained in step (1) were respectively used as immunogens to immunize mice, and the natural protein NT-proBNP (provided by Xinbainuo Biological Co., Ltd.) was selected to immunize mice As a control, an anti-first polypeptide monoclonal antibody, an anti-second polypeptide monoclonal antibody, an anti-third polypeptide monoclonal antibody and an anti-natural protein monocl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com