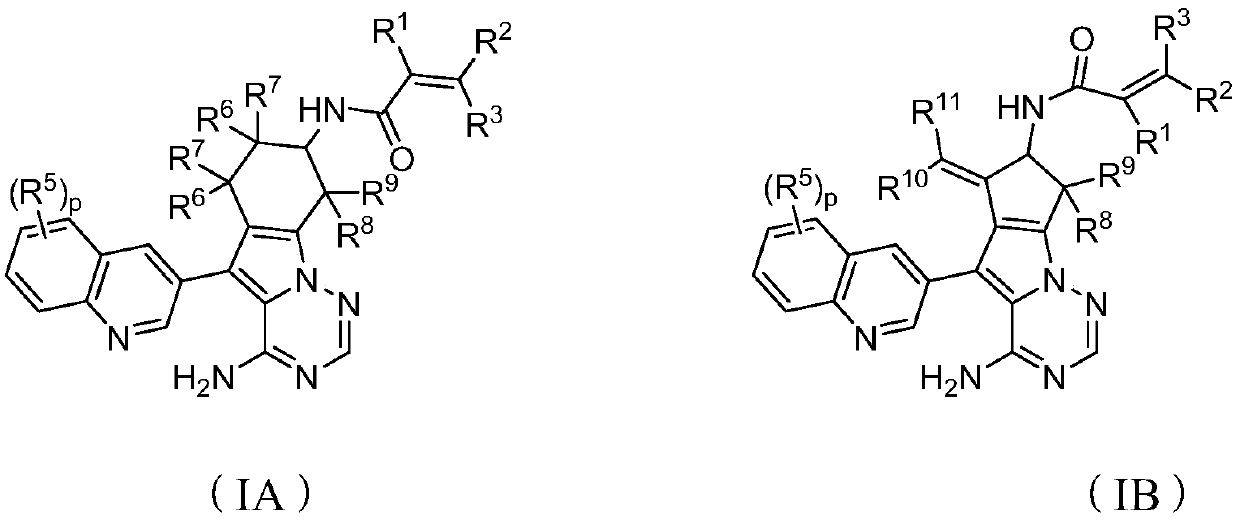
Preparation method and application of compound or its pharmaceutically acceptable salt or composition
A technology for compounds and medicinal salts, applied to the application field in the preparation of medicines, can solve the problems of no good treatment method, poor mutant effect, ineffective kinase activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] (S)-N-(4amino-5-(quinolin-3-yl)-6,7,8,9-tetrahydro-[1,2,4]triazine[1,6-a]indole -8-yl)acrylamide
[0066] Step 1. tert-butyl 1H-pyrrol-1-ylcarbamate
[0067]
[0068]In a 2L three-neck flask, add tert-butyl carbazate (100 g, 0.76 mol), 2,5-dimethoxytetrahydrofuran (108 g, 0.83 mol) and dioxane (700 mL). Under stirring, dilute hydrochloric acid (2M, 10 mL) was slowly added to the above solution, and then heated to 100° C. for 48 h. After the reaction was completed, the solvent was distilled off under reduced pressure, and the residue was dissolved in EtOAc (500 mL). The ester phase is sequentially saturated with Na 2 CO 3 solution, washed with saturated NaCl aqueous solution, anhydrous Na 2 SO 4 Dry, filter and concentrate to give a yellow solid. The obtained solid was dispersed in EtOH (100 mL), filtered, and the filter residue was washed with a small amount of EtOH, and dried to obtain 80 g of the target product. Yield 58%. LCMS(ESI):m / z=183(M+H) + .
[0...
Embodiment 2
[0113] (R)-N-(4amino-5-(quinolin-3-yl)-6,7,8,9-tetrahydro-[1,2,4]triazine[1,6-a]indole -8-yl)acrylamide
[0114] Step 1. Methyl (R)-2-((tert-butoxycarbonyl)amino)-3-iodopropionate
[0115]
[0116] Using N-Boc-L-serine methyl ester as a raw material, it is synthesized according to the method in Step 6 of Example 1. LCMS(ESI):m / z=352(M+Na) + .
[0117] Step 2. Methyl (S)-3-(4-aminopyrrole[1,2-f][1,2,4]triazin-7-yl)-2-(tert-butoxycarbonylamino)propionate
[0118]
[0119] According to the method of Step 7 of Example 1, it is synthesized from methyl (R)-2-((tert-butoxycarbonyl)amino)-3-iodopropionate. LCMS(ESI):m / z=336(M+H) + .
[0120] Step 3. Methyl(S)-3-(4-amino-5-iodopyrrole[1,2-f][1,2,4]triazin-7-yl)-2-(tert-butoxycarbonylamino ) propionate
[0121]
[0122] According to the method of step 8 of Example 1, from methyl (S)-3-(4-aminopyrrole[1,2-f][1,2,4]triazin-7-yl)-2-(tert-butoxy Carbonyl amino) propionate synthesized. LCMS(ESI):m / z=462(M+H) + .
[0123]...
Embodiment 3
[0145] (R)-N-(4-Amino-6-methylene-5-(quinolin-3-yl)-7,8-dihydro-6H-cyclopenta[4,5]pyrrole[2, 1-f][1,2,4]triazin-7-yl)acrylamide
[0146] Step 1. tert-butyl(R)-(4-amino-6-methylene-5-(quinolin-3-yl)-7,8-dihydro-6H-cyclopenta[4,5] Pyrrolo[2,1-f][1,2,4]triazin-7-yl)carbamate
[0147]
[0148] In a 50mL round bottom flask, under nitrogen protection, add tert-butyl (R)-(1-(4-amino-6-iodo-5-(quinolin-3-yl)pyrrole[1,2-f] [1,2,4]Triazin-7-yl)-but-3-en-2-yl)carbamate (1.0g, 1.8mmol), AcOK (353mg, 3.6mmol), pd(dppf)Cl 2 (131mg, 0.18mmol) and DMF (10mL), heated to 80°C for 1h. After the reaction was completed, water was added and extracted with EtOAc. The organic phase was washed with saturated NaCl solution, anhydrous Na 2 SO 4 Dry, filter and concentrate. The resulting residue was purified by silica gel column chromatography (CH 2 Cl 2 : MeOH (v / v)=40:1) to obtain 500 mg, yield 65%. LCMS(ESI):m / z=429(M+H) + .
[0149] Step 2. (R)-6-Methylene-5-(quinolin-3-yl)-7,8-dihydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com