Method for synthesizing houttuynin Schiff base and copper complex thereof
A technology of houttuyfonate and copper complexes, which is applied in the preparation of copper organic compounds, imino compounds, organic chemistry, etc., can solve the problems of rare articles, and achieve the effects of convenient operation, high yield and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Step 1. Synthesis of Schiff base C 24 h 44 o 2 N 2
[0053] Ⅰ. Dissolve 0.2740g (1mmol) sodium octanoyl acetaldehyde sulfite in 60mL of 5%-20% ethanol solution, condense and reflux magnetically for 0.5h under the condition of heating in a water bath. g 1,4-Butanediamine (1 mmol). 40°C water bath condensation reflux magnetic stirring 0.5-3h.
[0054] II. Cool the reaction solution in step I to room temperature, filter, and wash the filter cake three times with distilled water. The filter cake was vacuum-dried to obtain Schiff's base. The yield is about 89%.
[0055] (1)C 24 h 44 o 2 N 2 Infrared characterization of
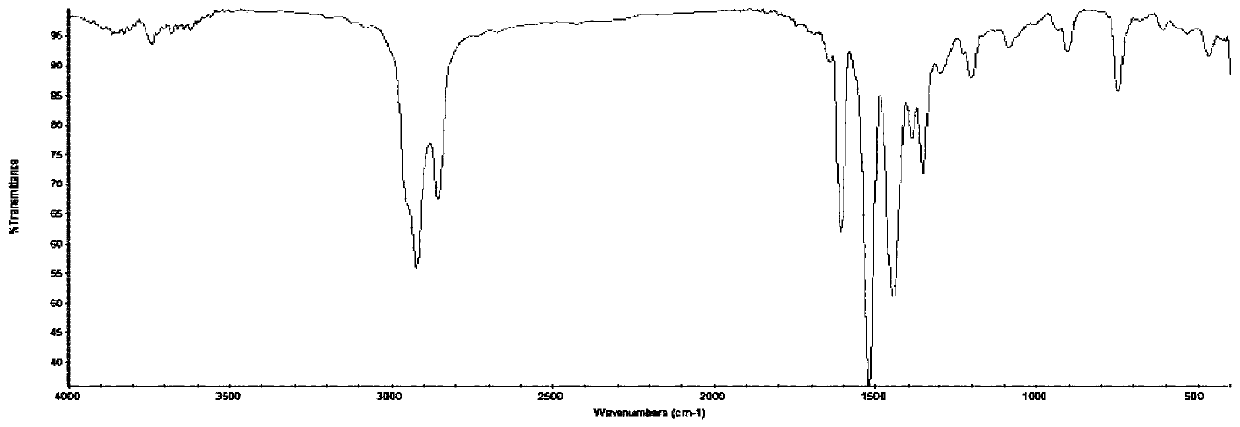
[0056] C 24 h 44 o 2 N 2 For the infrared spectrum, see figure 1 (Instrument model: NEXUS infrared spectrometer). Compared with the raw material octanoylacetaldehyde sodium sulfite, C 24 h 44 o 2 N 2 In the infrared spectrum, at 1646cm -1 A new peak appears at , which is the stretching vibration peak of C=N, indicating the formation of...
Embodiment 2
[0066] Step 1. Synthesis of Schiff base C 28 h 52 o 2 N 2
[0067] Ⅰ. Dissolve 0.3020g (1mmol) houttuyfonate sodium in 60mL of 5%-20% ethanol solution, condense and reflux magnetically for 0.5h under the condition of heating in a water bath. 0.0882g 1,4-Butanediamine (1mmol). Condensate in a water bath at 40°C and stir magnetically for 0.5-3h.
[0068] II. Cool the reaction solution in step I to room temperature, filter, and wash the filter cake three times with distilled water. The filter cake was vacuum-dried to obtain Schiff's base. The yield is about 88%
[0069] (1)C 28 h 52 o 2 N 2 Infrared characterization of
[0070] C 28 h 52 o 2 N 2 For the infrared spectrum, see image 3 (Instrument model: NEXUS infrared spectrometer). Compared with raw material houttuyfonate sodium, C 28 h 52 o 2 N 2 In the infrared spectrum, at 1647cm -1 A new peak appears at , which is the stretching vibration peak of C=N, indicating the formation of a Schiff base; 1036cm -...
Embodiment 3
[0079] Step 1. Synthesis of Schiff base C 32 h 60 o 2 N 2
[0080] Ⅰ. Dissolve 0.3300g (1mmol) neohouttuyfonate sodium in 60mL of 5%-20% ethanol solution, condense and reflux magnetically for 0.5h under the condition of heating in a water bath. 0.0882 g of 1,4-butanediamine (1 mmol) was added dropwise. Condensation and reflux magnetic stirring for 0.5-3h under the condition of water bath heating.
[0081] II. Cool the reaction solution in step I overnight, filter, and wash the filter cake three times with distilled water. The filter cake was vacuum-dried to obtain Schiff's base. The yield is about 86%
[0082] (1)C 32 h 60 o 2 N 2 infrared characterization. (Instrument model: NEXUS infrared spectrometer)
[0083] C 32 h 60 o 2 N 2 For the infrared spectrum, see Figure 5 .C 32 h 60 o 2 N 2 In the infrared spectrum, at 1647cm -1 A new peak appears at , which is the stretching vibration peak of C=N, indicating the formation of a Schiff base; 1040cm -1 , 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com