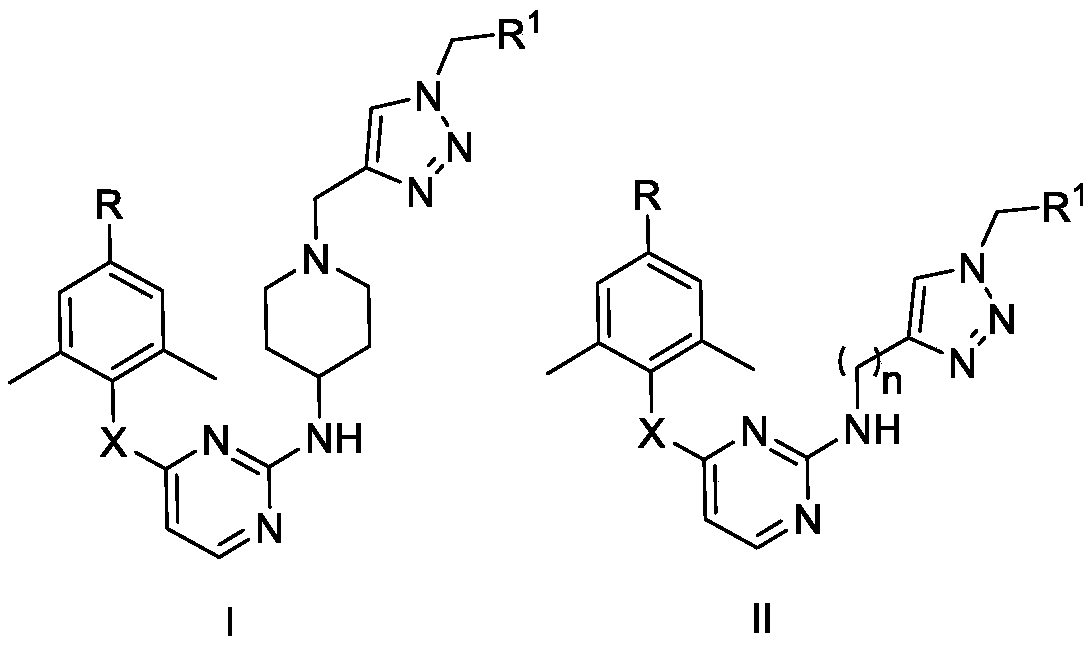
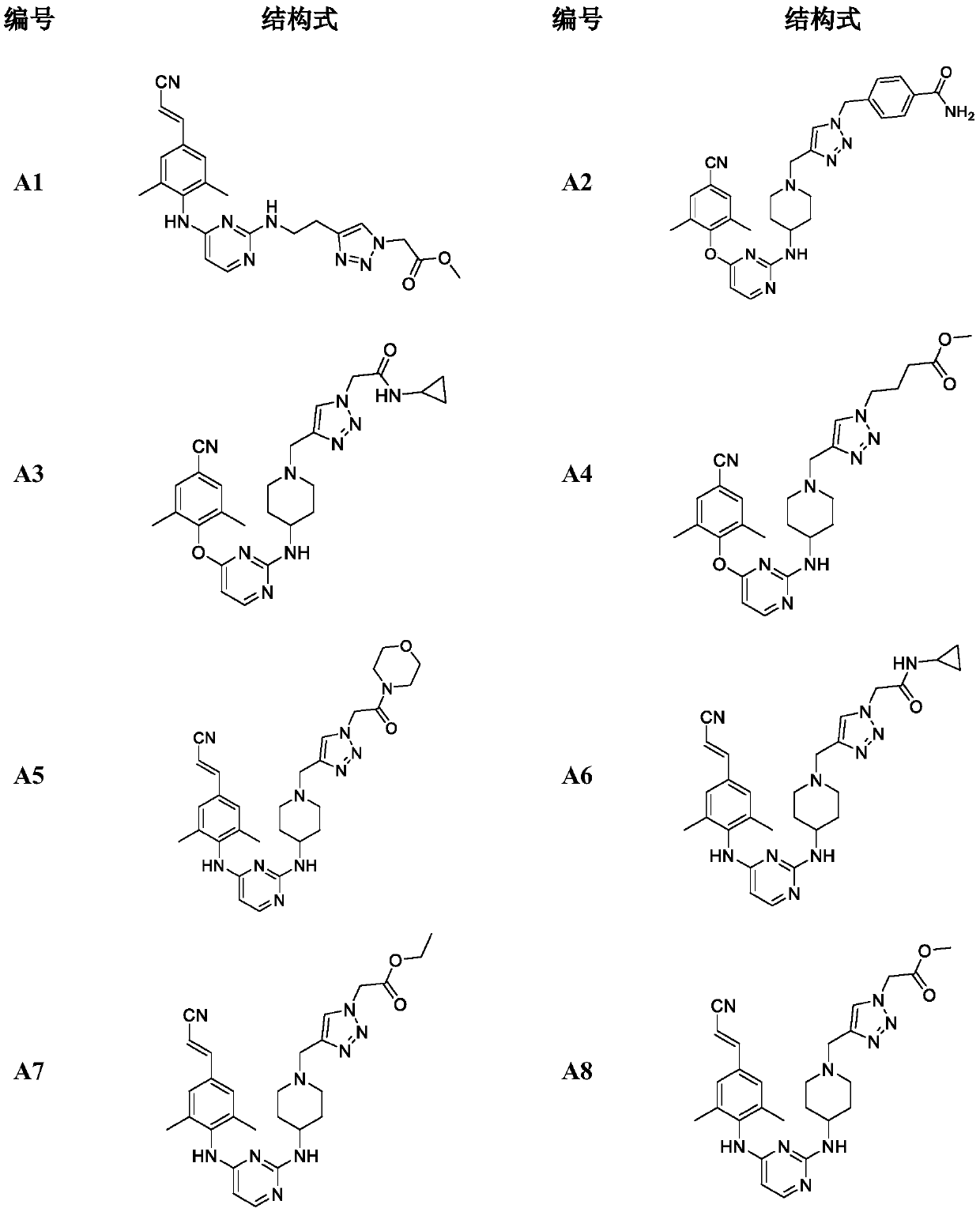
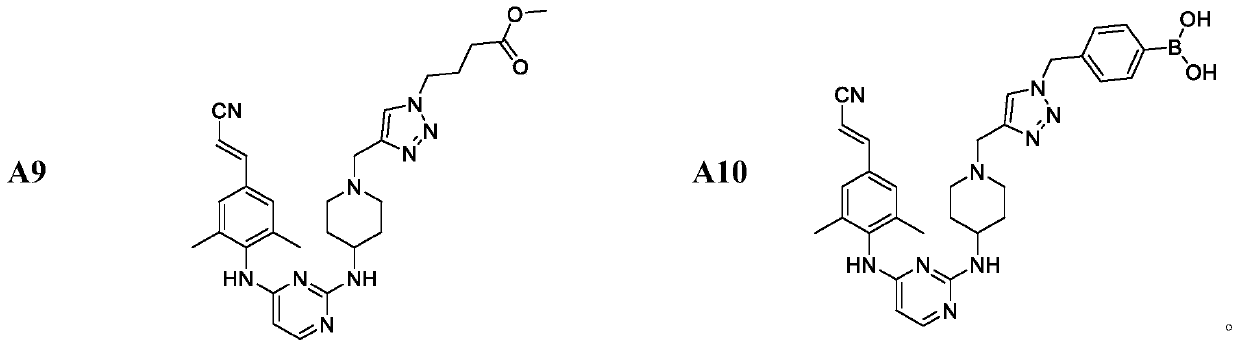
Monoaromatic miazine HIV-1 reverse transcriptase inhibitor containing triazole rings and preparation method and application thereof
A technology for reverse transcriptase inhibition and HIV-1, applied to medical preparations containing active ingredients, chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, etc., can solve toxic side effects, poor solubility, etc. problem, to achieve the effect of high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Preparation of 4-((2-chloropyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (7)
[0045]
[0046] Starting materials 2,4-dichloropyrimidine (13.42mmol, 2.00g), 3,5-dimethyl-4-hydroxybenzonitrile (14.76mmol, 2.18g) and potassium carbonate (16.10mmol, 2.22g) were added to 20mL N,N-dimethylformamide (DMF) solution, stirred at room temperature, and reacted for 8-12h. The reaction was monitored by TLC. After the reaction was completed, 40 mL of aqueous solution was added to the reaction solution and stirring was continued for 20-30 min, and a white solid, Intermediate 7, was obtained by filtration. 1 H NMR (400MHz, DMSO-d 6 )δ8.70(d, J=5.7Hz, 1H), 7.75(s, 2H), 7.32(d, J=5.7Hz, 1H), 2.10(s, 6H); ESI-MS: m / z 259.97( M+H) + ,C 13 h 10 ClN 3 O(259.05).
Embodiment 2
[0047] Example 2: Preparation of 4-((2-chloropyrimidin-4-yl)amino)-3,5-dimethylbenzaldehyde (11)
[0048]
[0049] The operation steps are the same as the preparation of intermediate 7, the difference is that 3,5-dimethyl-4-hydroxy-benzaldehyde is used to replace 3,5-dimethyl-4-hydroxybenzonitrile in the preparation process of 7 to obtain white solid. 1 H NMR (400MHz, DMSO-d 6 )δ9.97(s,1H),8.70(d,J=5.7Hz,1H),7.77(s,2H),7.30(d,J=5.7Hz,1H),2.15(s,6H); ESI- MS:m / z263.10(M+H) + ,C 13 h 11 ClN 2 o 2 (262.05).
Embodiment 3
[0050] Example 3: Preparation of (E)-3-(4-((2-chloropyrimidin-4-yl)amino)-3,5-dimethylphenyl)acrylonitrile (12)
[0051]
[0052] Under ice-bath conditions, diethyl cyanomethylphosphate (11.40mmol, 2.02g) was added to 10mL of a mixed solution of tetrahydrofuran and dichloromethane (v:v=1:1), and after thorough mixing, the Potassium tert-butoxide (11.40 mmol, 1.28 g) was added. Then, intermediate 11 (3.81 mmol, 1.00 g) dissolved in 5 mL of tetrahydrofuran was slowly added dropwise to the above mixed solution. After the dropwise addition was completed, the ice bath was removed and the reaction was carried out at room temperature. After the reaction was completed, the solvent was removed under reduced pressure, dissolved in 20 mL of DMF, and then 60-80 mL of aqueous solution was added to precipitate a white solid. Filtrate, wash the filter cake with water, and recrystallize from chloroform to obtain intermediate (E)-3-(4-((2-chloropyrimidin-4-yl)amino)-3,5-dimethylphenyl)acry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com