Plasmid carrier pair and immune cells modified by same, and application thereof
A plasmid carrier and immune cell technology, applied to genetically modified cells, cells modified by introducing foreign genetic material, blood/immune system cells, etc., can solve the problems that do not mention the necessity and beneficial effects of IL-12 , to avoid toxic side effects, reduce toxicity and enhance the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
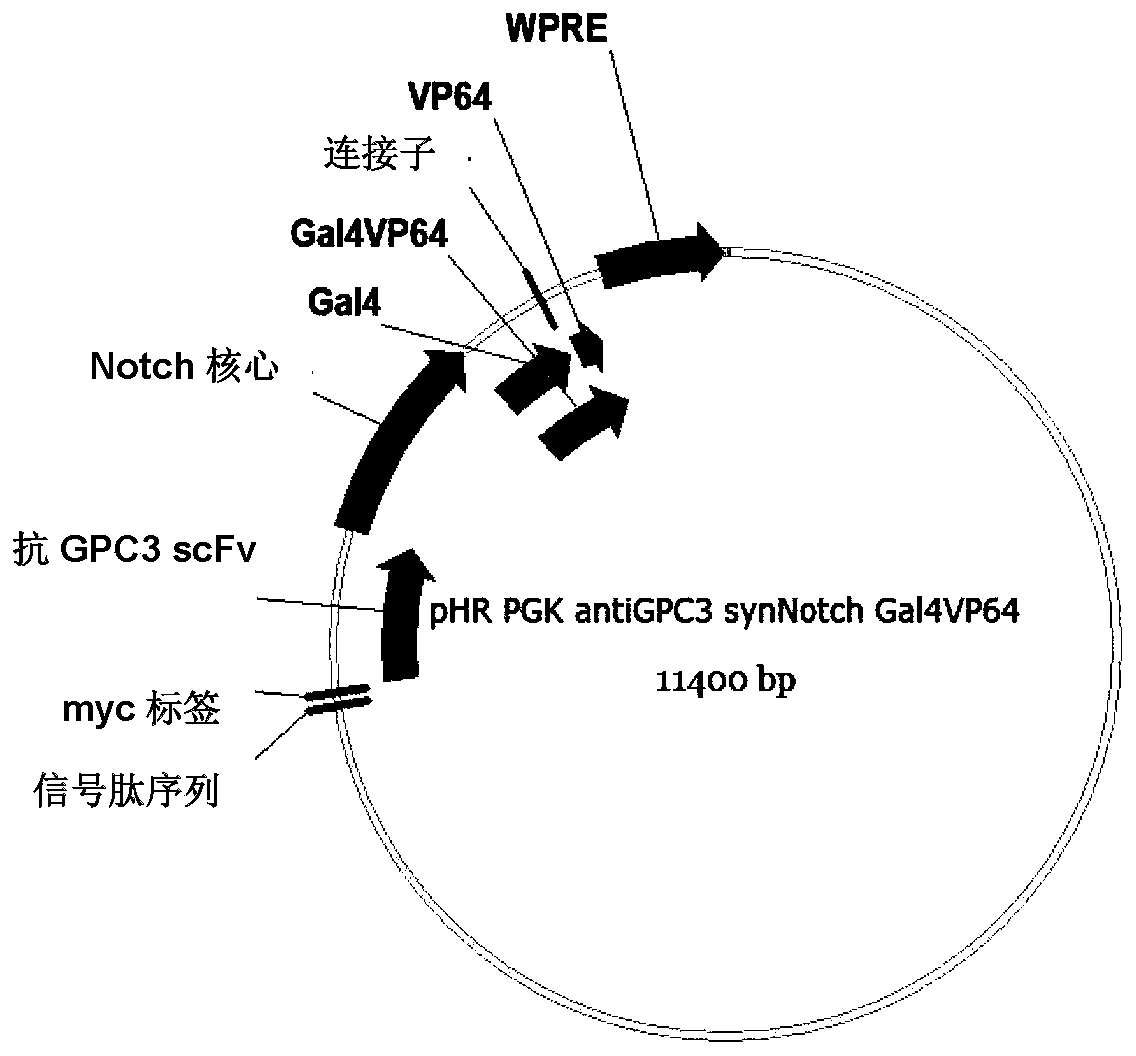
[0107] Example 1: Construction of lentiviral plasmid and viral packaging of chimeric antigen receptor protein encoded by nucleic acid
[0108] Table 1 below illustrates the linking sequence of the various parts of the chimeric antigen receptor of the example of the present invention.
[0109] Table 1 Connection sequence of each part of chimeric antigen receptor
[0110]
[0111] 1. AntiGPC3-synNotch-GAL4VP64 nucleic acid fragment amplification
[0112] 1) Amplification of GPC3 scFv sequence
[0113] Obtain the nucleic acid sequence (SEQ ID NO: 2) of the scFv of antiGPC3 by conventional PCR method
[0114] Other nucleic acid sequences were obtained by PCR using PHR-PGK-antiCD19-synNotch-GAL4VP64 (purchased from addgene) as a template. Wherein the signal sequence (SEQ ID NO:1) is represented by a primer pair [upstream primer: 5'-tctcacgcgtcaagtggagc-3'(SEQ ID NO:14), downstream primer: 5'-tctgcaccagctgcacctcgaggtcctcttcagag-3'(SEQ ID NO:15)] PCR amplification was carried ...
Embodiment 2
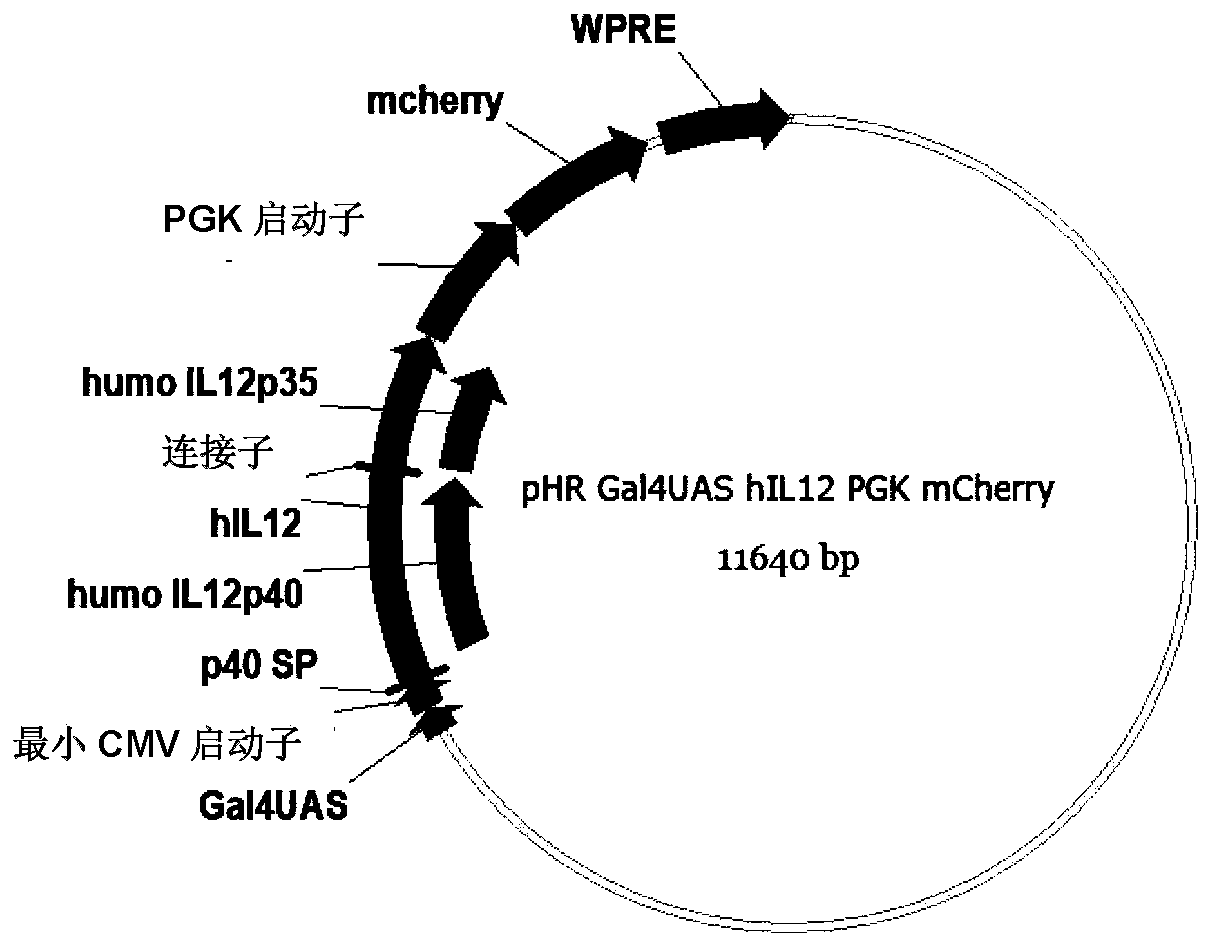
[0148] Example 2: Infection of NK92 cells with recombinant lentivirus
[0149] Infect NK92 cells with the lentivirus 1 prepared in Example 1 to obtain GPC3-SYN-IL12-NK92 cells, the specific operations are as follows:
[0150] 1) The day before infection, coat a 24-well plate with recombinant human fibronectin (Retronectin), add 380 μl of 5 μg / ml recombinant human fibronectin solution (PBS) to each well, and incubate overnight at 4°C;
[0151] 2) Discard the recombinant human fibronectin solution (PBS) in the 24-well plate during infection, and wash twice with 1 ml of PBS. Infect with the above-mentioned recombinant lentivirus at MOI=30, and at the same time add polybrene at a final concentration of 10 μg / ml to improve the infection efficiency, and the number of cells per well is 5×10 5 , culture medium 500μl, 32°C, 1800g, after centrifugation for 90min, transfer to the cell culture incubator;
[0152] 3) Cells after infection were treated with 5×10 cells every other day 5 / ...
Embodiment 3
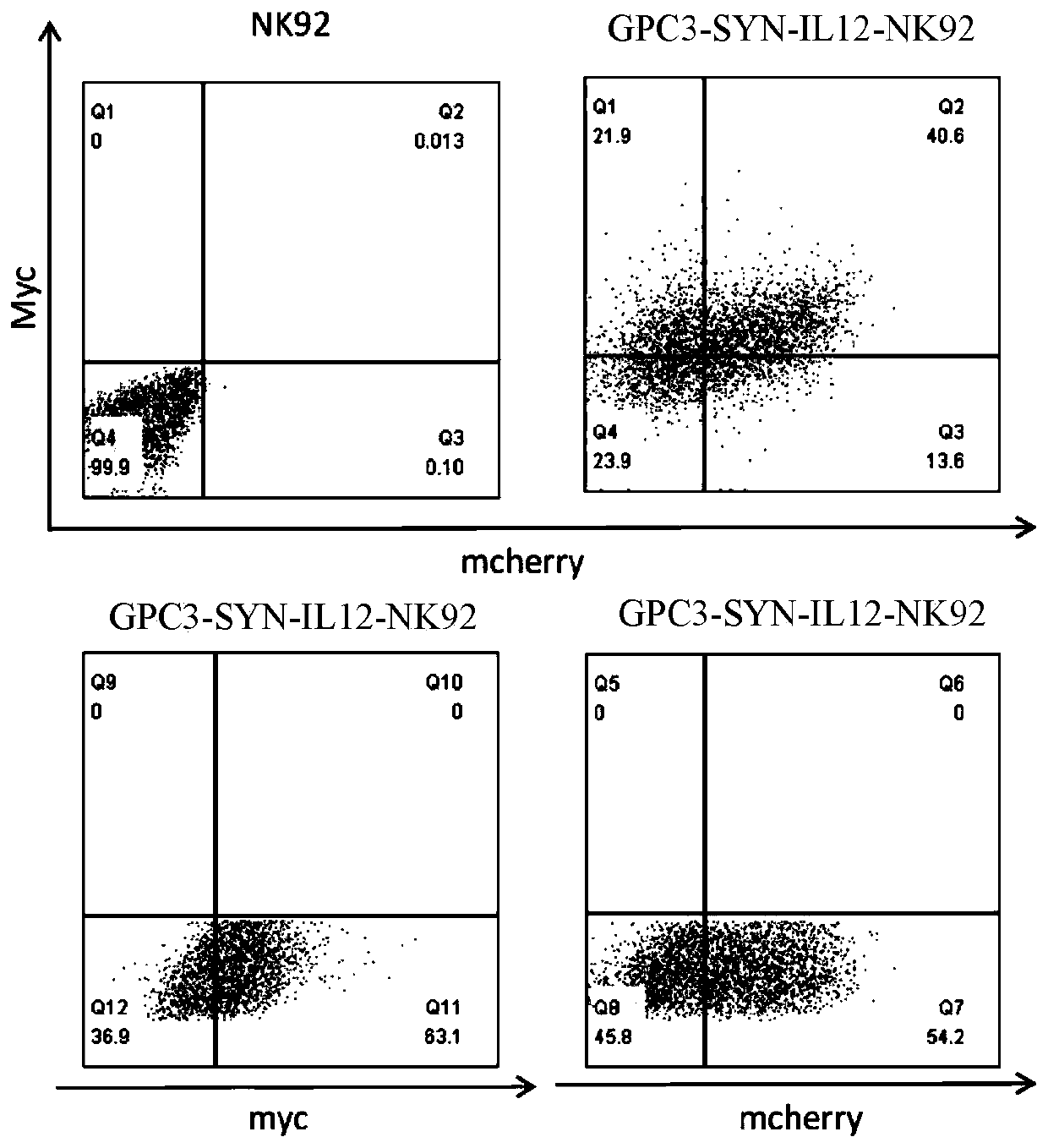
[0153] Example 3: Identification of GPC3-SYN-IL12-NK92 cells
[0154] On the 7th day of culture, the lentivirus-infected NK92 cells were detected by flow cytometry to detect the expression of different receptors. Since the N-terminus of antiGPC3 has a Myc tag, the detection of Myc expression is a positive cell successfully infected by pHRLSIN-antiGPC3-synNotch-GAL4VP64, Detecting the expression of mcherry means the positive cells successfully infected by pHRLSIN-GAL4UAS-IL12-PGK-mcherry, and the simultaneous expression of Myc and mcherry means the positive cells successfully double infected by GPC3-SYN-IL12-NK92.
[0155] 1) 1×10 NK92 cells infected with different 6 Divide the cells into 2ml centrifuge tubes, centrifuge at 5000rpm at 4°C for 5min, discard the supernatant, and wash twice with PBS;
[0156] 2) The cells in the control group for detection of myc expression were washed twice with direct PBS (2% NBS) and resuspended as a control; the cells in the detection group w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com