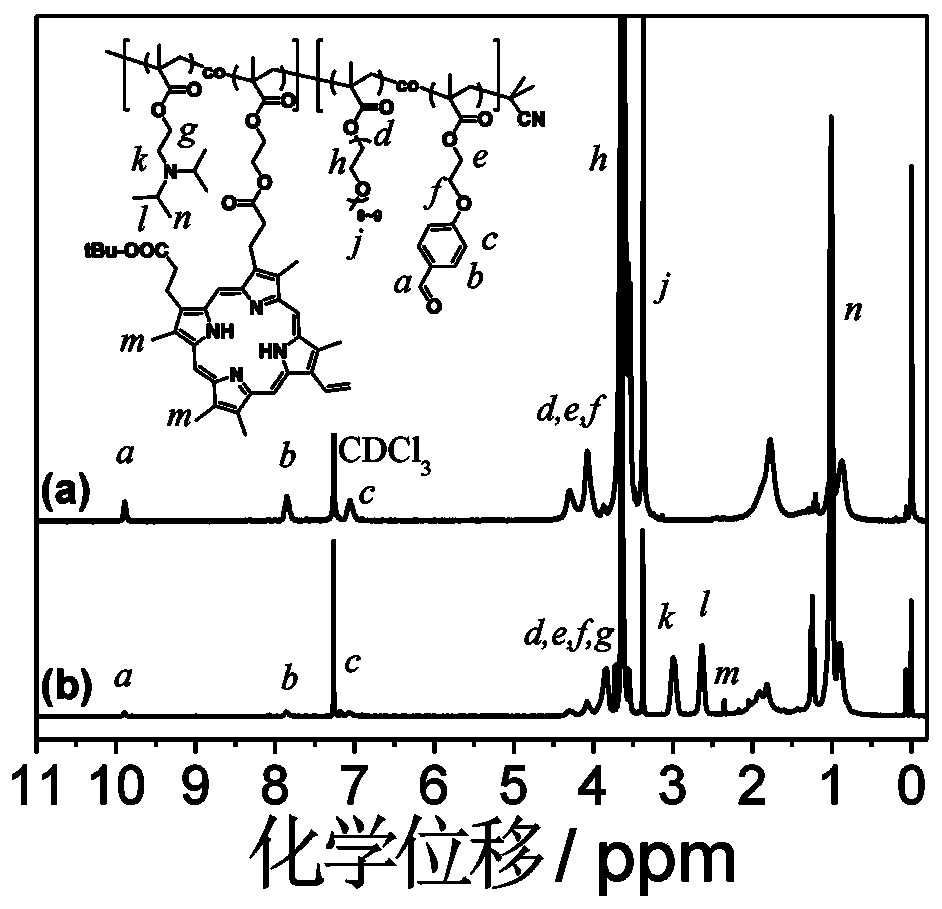
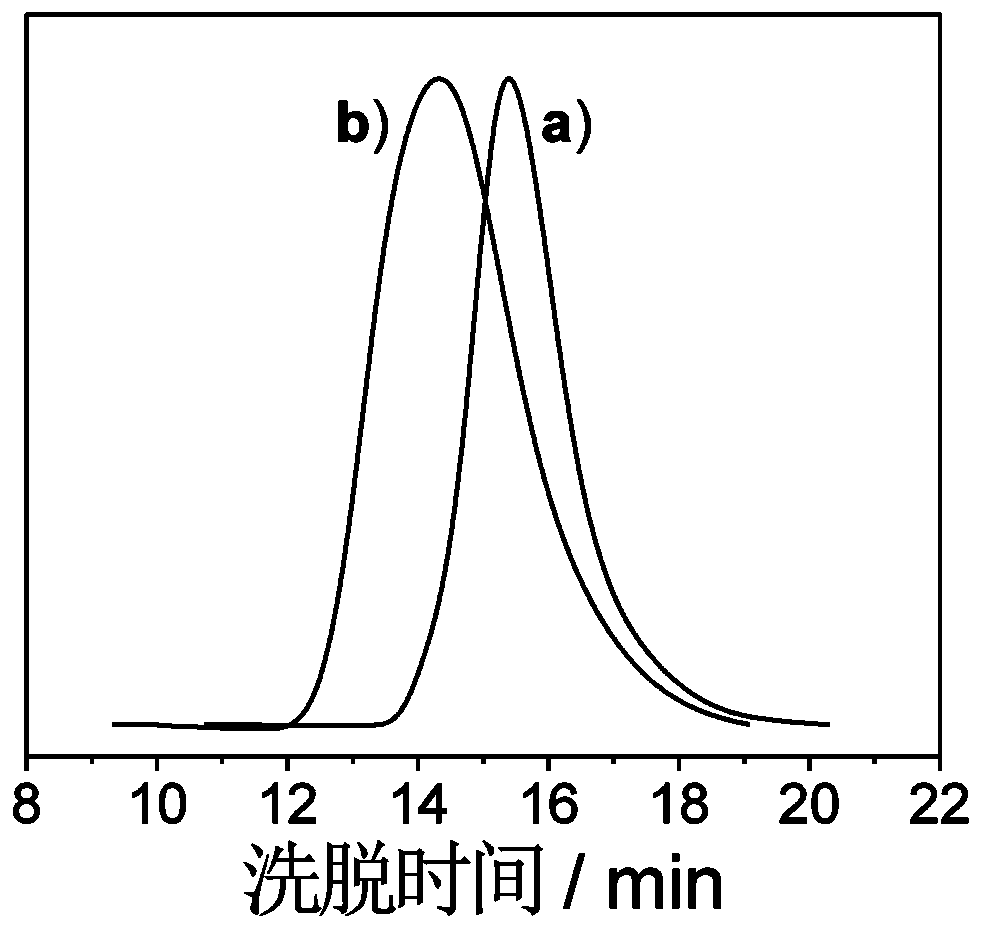
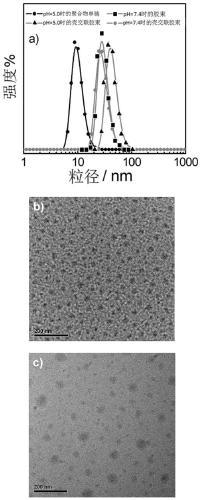
Amphiphilic segmented copolymer as well as shell crosslinking micelle, preparation method and application thereof
An amphiphilic block and copolymer technology, which can be used in pharmaceutical formulations, medical preparations with inactive ingredients, and emulsion delivery, etc., and can solve problems such as block copolymer assembly damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0107] The first step is to prepare ABEMA with aldehyde functional groups:
[0108]
[0109] Its characteristics are: the monomer can be well copolymerized with the hydrophilic monomer oligoethylene glycol methacrylate to obtain a hydrophilic segment, and the side group of the monomer contains an aldehyde functional group, which can be combined with The shell cross-linking agent 9,10-aminoethoxyanthracene undergoes a cross-linking reaction, and finally a shell-cross-linked nanomicelle assembly is obtained.
[0110] Preparation:
[0111] Dissolve 2.44g of 4-hydroxybenzaldehyde (Chinese medicine) in 50mL of acetone, then add potassium carbonate (4.146g, 30mmol, Chinese medicine) and bromoethanol (3.75g, 30mmol, Chinese medicine) to the system, and reflux overnight. Then filter to remove inorganic salts and remove acetone by rotary evaporation, then extract after dissolving with dichloromethane, keep the organic phase, remove dichloromethane by rotary evaporation, and finally...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com