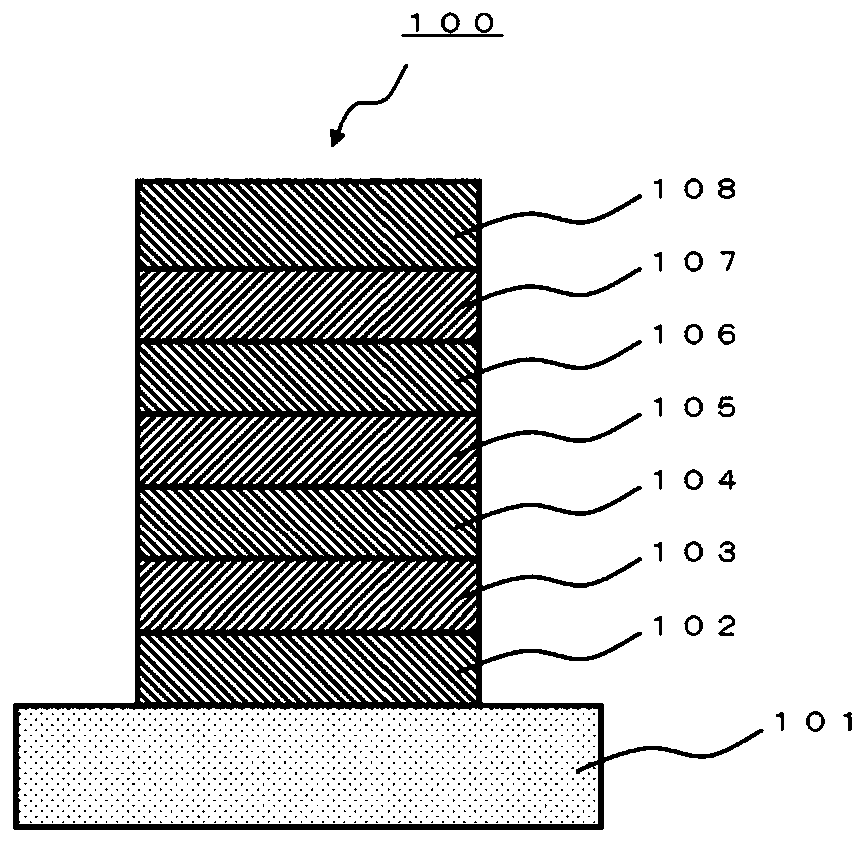
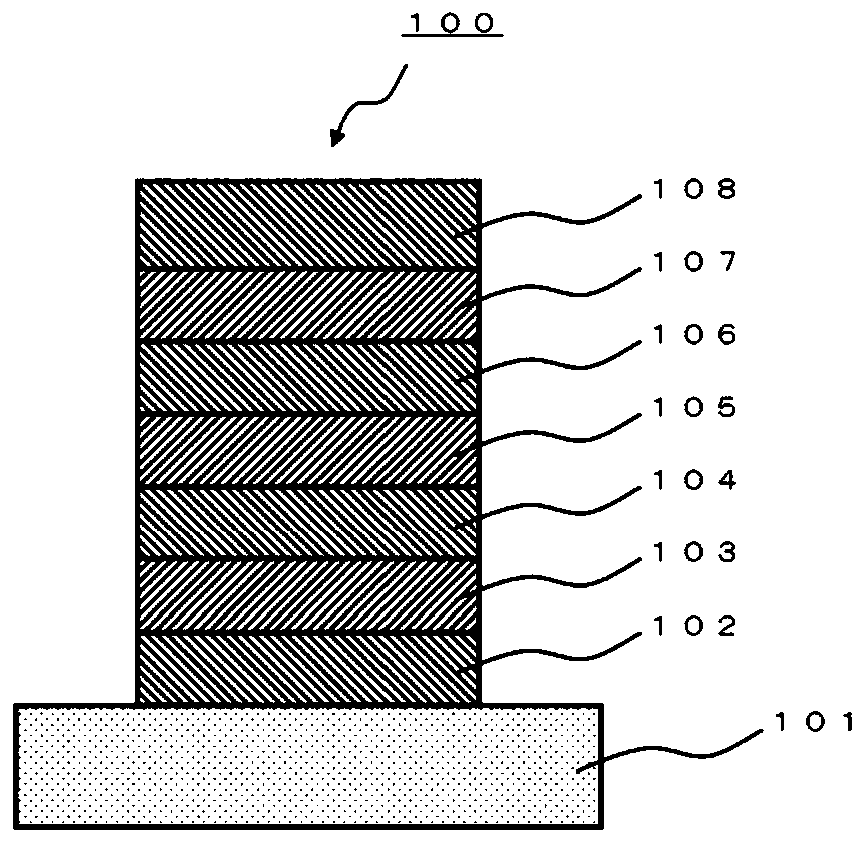
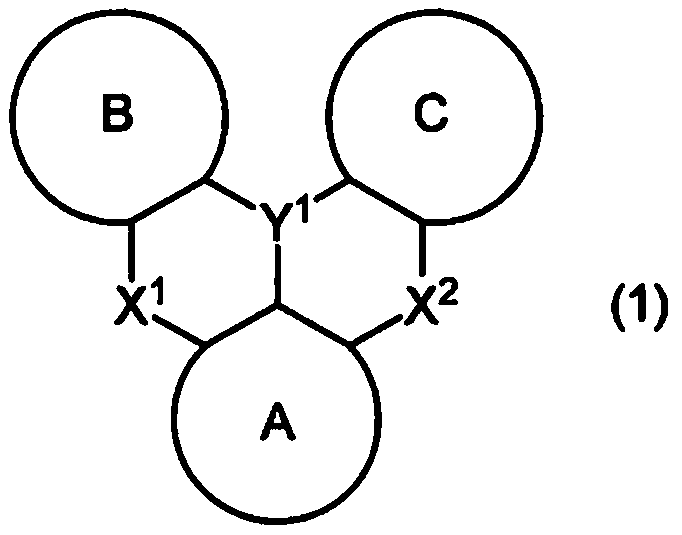
A polycyclic aromatic compound and a polymer thereof, a material for an organic element, an organic electroluminescent element, a display device, or a lighting device.
A polycyclic aromatic compound technology, applied in the field of polycyclic aromatic compounds and their polymers, can solve the problems of insufficient aromatic epoxidation-reduction stability, insufficient life, and unsuitable main materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0675] Hereinafter, although an Example demonstrates this invention more concretely, this invention is not limited to these. First, a synthesis example of a polycyclic aromatic compound will be described below.
Synthetic example (1
[0676] Synthesis Example (1): Synthesis of Compound (1-22)
[0677] [Chemical 122]
[0678]
[0679] Under nitrogen atmosphere, 3,4,5-trichloroaniline (12.0 g), d 5 -Bromobenzene (30.0 g), dichlorobis[(di-tert-butyl(4-dimethylaminophenyl)phosphino)]palladium (Pd-132, 0.43 g) as a palladium catalyst, tert-butanol Sodium (NaOtBu, 14.7 g) and xylene (200 ml) were put into the flask and heated at 120° C. for 3 hours. After the reaction, water and ethyl acetate were added to the reaction liquid and stirred, and then the organic layer was separated and washed with water. Then, the organic layer was concentrated to obtain a crude product. The crude product was purified with a silica gel short-path column (eluent: toluene / heptane=1 / 1 (volume ratio)) to obtain Intermediate (I-A) (15.0 g).
[0680] [Chemical 123]
[0681]
[0682]Under nitrogen atmosphere, intermediate (I-A) (15.0 g), bis(4-tert-butylphenyl)amine (25.9 g), bis(dibenzylideneacetone)palladium (0.48 g), 2-diphen...
Synthetic example (2
[0690] Synthesis Example (2): Synthesis of Compound (1-102)
[0691] [Chemical 126]
[0692]
[0693] Under nitrogen atmosphere, the d 5 - Aniline (5.0g), d 5 -Bromobenzene (8.25 g), Pd-132 (0.36 g) as a palladium catalyst, NaOtBu (7.1 g) and xylene (100 ml) were put into a flask, and heated at 120° C. for 1.5 hours. After the reaction, water and ethyl acetate were added to the reaction liquid and stirred, and then the organic layer was separated and washed with water. Then, the organic layer was concentrated to obtain a crude product. The crude product was purified with a silica gel short-path column (eluent: toluene / heptane=1 / 1 (volume ratio)) to obtain Intermediate (I-C) (8.1 g).
[0694] [Chemical 127]
[0695]
[0696] Under nitrogen atmosphere, intermediate (I-C) (8.0 g), intermediate (I-D) (20.6 g), Pd-132 (0.31 g) as a palladium catalyst, NaOtBu (6.4 g) and xylene (100 ml) were put together into a flask and heated at 120°C for 1 hour. After the reaction, w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electron work function | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com