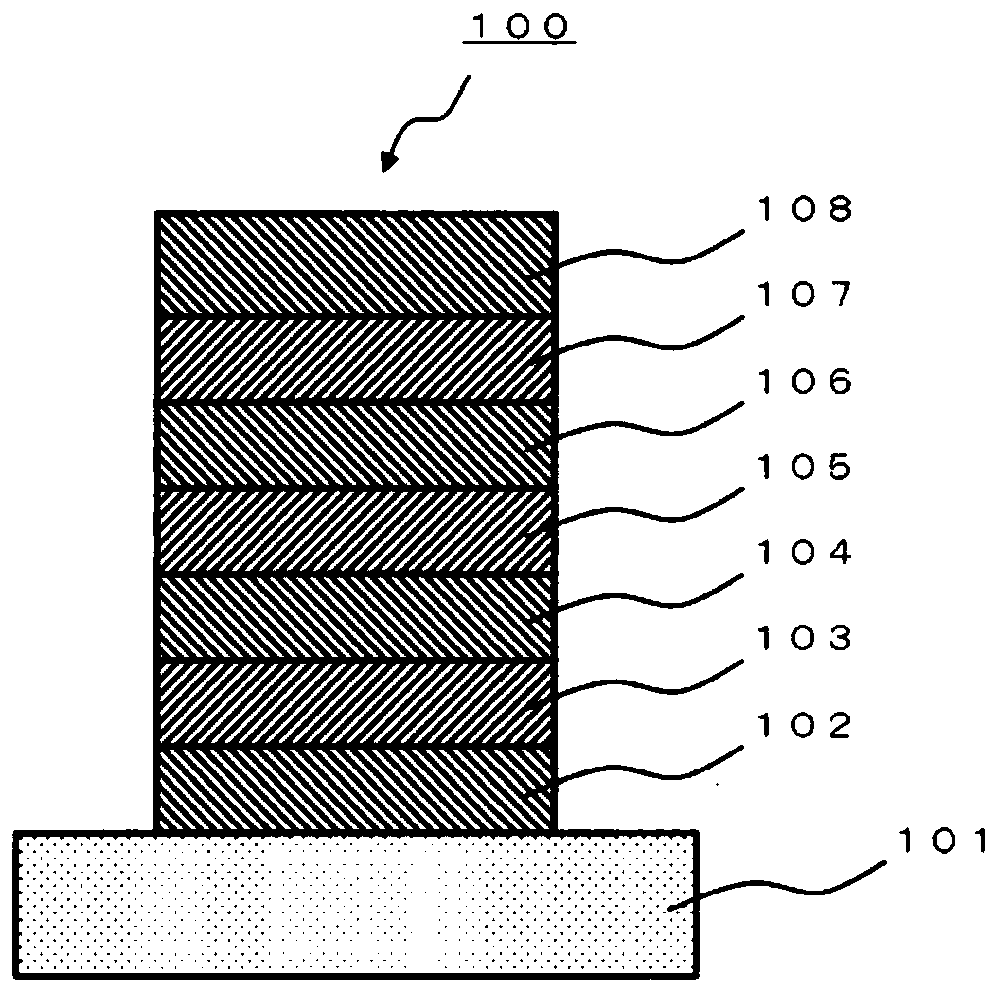
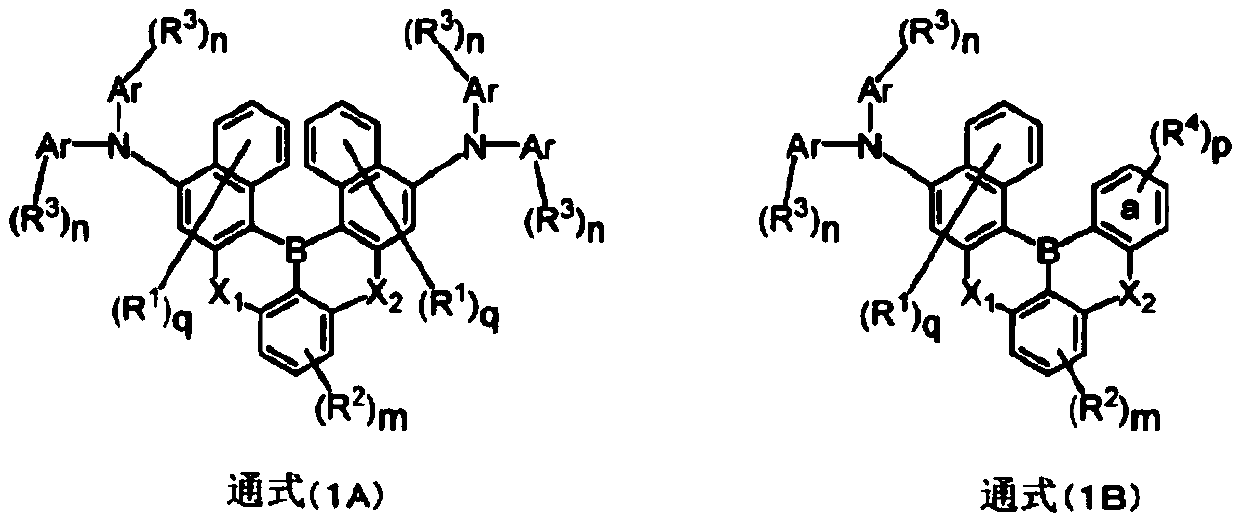
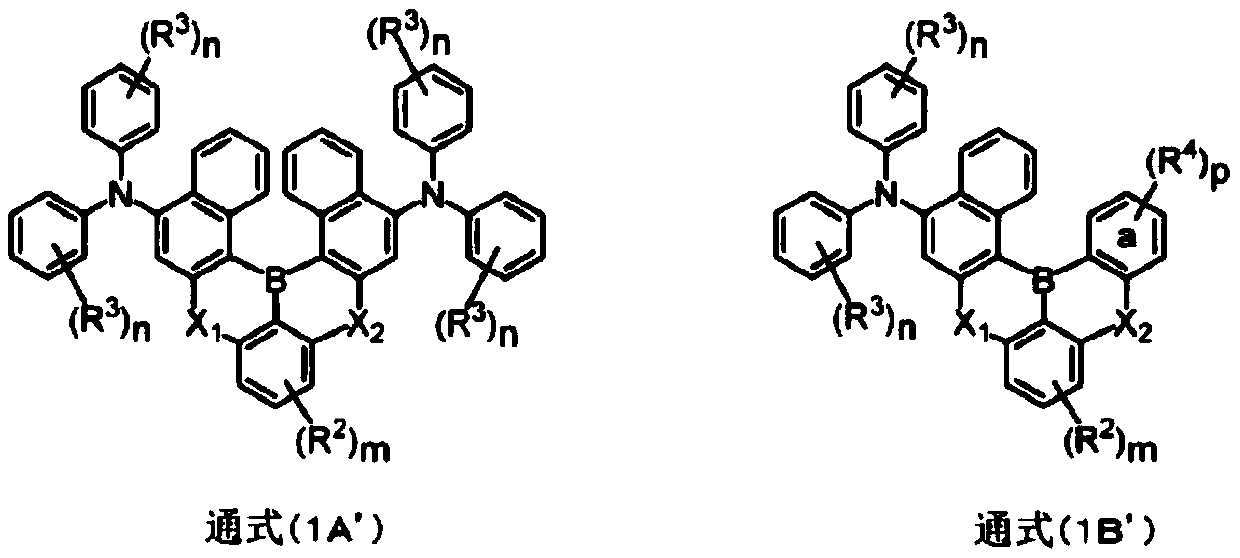
Polycyclic aromatic amino compound
An amino compound and polycyclic aromatic technology, applied in the field of polycyclic aromatic amino compounds, can solve the problems of unsuitable main material, insufficient life, insufficient redox stability of aromatic epoxies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0419] Hereinafter, although an Example demonstrates this invention more concretely, this invention is not limited to these Examples. First, a synthesis example of a polycyclic aromatic amino compound will be described below.
Synthetic example (1
[0421] Compound of formula (1A-1): N 5 ,N 5 ,N 13 ,N 13 -Synthesis of tetraphenyl-7,11-dioxa-17c-boraphenanthrene[2,3,4-no]tetraphenol-5,13-diamine
[0422] [chem 94]
[0423]
[0424]
[0425] Under nitrogen atmosphere, put diphenylamine (22.3g), 4-bromonaphthalene-2-ol (28.0g), Pd-132 (Johnson Matthey) (0.9g), NaOtBu (30.0g) and toluene (252ml) were heated and refluxed for 4 hours. After cooling the reaction liquid to room temperature, water and ethyl acetate were added and liquid-separated. Further, purification was carried out with a silica gel column (eluent: toluene) to obtain 35 g of 4-(diphenylamino)naphth-2-ol as an intermediate compound (yield: 89.5%).
[0426] [chem 95]
[0427]
[0428]
[0429] Under a nitrogen atmosphere, put 4-(diphenylamino)naphthalene-2-ol (16.0g), 2-bromo-1,3-difluorobenzene (5.0g), potassium carbonate (17.8g) and A flask containing 1-methyl-2-pyrrolidone (30 ml) was heated and stirred at reflux temperature for 8 hours. Aft...
Synthetic example (2
[0443] Compounds of formula (1A-173): N 5 ,N 5 ,N 13 ,N 13 ,Synthesis of 8,10-hexaphenyl-7,11-dioxa-17c-boraphenanthrene[2,3,4-no]tetraphenol-5,13-diamine
[0444] [chem 99]
[0445]
[0446]
[0447] Under nitrogen atmosphere, add 4-(diphenylamino)naphthalene-2-ol (16.1g), 5'-bromo-4',6'-difluoro-1,1':3',1 A flask of "-terphenyl (8.5g), potassium carbonate (13.6g) and 1-methyl-2-pyrrolidone (43ml) was heated and stirred at reflux temperature for 3 hours. After the reaction was stopped, the reaction solution was cooled to At room temperature, the precipitate precipitated after adding water was extracted by suction filtration. After the obtained precipitate was washed with water and methanol in sequence, it was washed with a silica gel short path column (eluent: toluene) ) for purification. Next, reprecipitate with ethyl acetate to obtain 3,3'-((5'-bromo-[1,1':3',1"-terphenyl]- 4',6'-diyl)bis(oxy))bis(N,N-diphenylnaphthalen-1-amine) 22.6 g (yield: 98.9%).
[0448] [...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electron work function | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com