Method for removing 1,1-difluoro-2-chloroethylene in 1,1,2,2-tetrafluoroethane by adsorption
A technology of tetrafluoroethane and vinyl chloride, which is applied in chemical instruments and methods, organic chemistry, preparation of halogenated hydrocarbons, etc., can solve the problems of high adsorption temperature and poor regeneration performance, and achieve large adsorption capacity and superior regeneration performance , fast adsorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
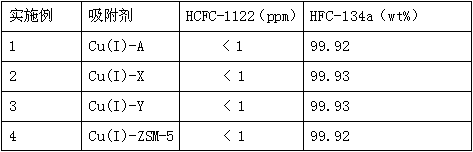
[0026] Example 1-4 The effect of molecular sieve type on the adsorption performance of Cu(I) ion modified molecular sieve
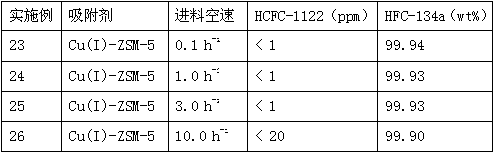
[0027] 10g of adsorbent is filled in a stainless steel tube with an inner diameter of 15 mm and a length of 150 mm, and then the stainless steel tube is embedded in an adsorption fixed bed, and the crude product of HFC-134a containing about 950 ppm of HCFC-1122 and with a purity of about 99.87% is separated into gas phase form, at a temperature of 40°C and a pressure of 1 atm, take 1.0h -1 The space velocity enters the adsorption fixed bed from the top of the adsorption fixed bed, and then the gas adsorbed by the adsorbent of the stainless steel tube is used to analyze the content of each component in it with a gas chromatograph. The results are shown in Table 1.
[0028] Cu(I) ion-modified molecular sieve adsorption performance result table of table 1 embodiment 1-4
[0029]
[0030] As can be seen from Table 1, the Cu(I) ion-modified A, X, Y and ZSM...
Embodiment 5-10
[0031] Example 5-10 Effect of Copper Source on Adsorption Performance of Cu(I) Ion Modified Molecular Sieve
[0032] Other conditions are the same as in Examples 1-4, except that the adsorbent is a Cu(II) ion-modified molecular sieve prepared by exchanging and modifying self-reduction by preparing Cu(II) solutions with different copper sources. The results are shown in the table 2.
[0033] Cu(I) ion-modified molecular sieve adsorption performance result table of table 2 embodiment 5-10
[0034]
[0035] It can be concluded from Table 2 that different types of adsorbents modified by different copper sources have strong adsorption capacity and can achieve better adsorption effect.
Embodiment 11-18
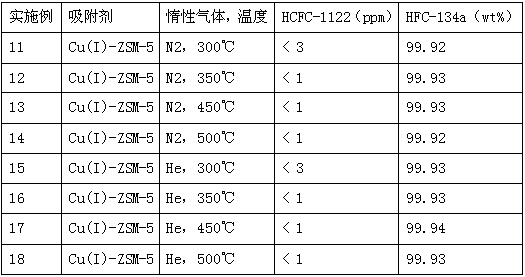
[0036] Examples 11-18 Influence of the type of inert gas and activation temperature on the adsorption performance of Cu(I) ion-modified molecular sieves
[0037] Other conditions are the same as in Examples 1-4, except that the type and temperature of the activated inert gas are different during the preparation of the adsorbent. The results are shown in Table 3.
[0038] Table 3 Adsorption performance results of Cu(I) ion-modified molecular sieves in Examples 11-18
[0039]
[0040] As can be seen from Table 3, under the same type of adsorbent of the present invention and the same activation temperature, He, N 2 It has strong adsorption capacity when activated as an inert gas; the same type of adsorbent and the same activated inert gas have strong adsorption capacity at different activation temperatures (300°C, 350°C, 450°C, 500°C). , although when N2 is activated as an inert gas at an activation temperature of 300°C, compared with other temperatures, the adsorption capaci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com