Preparation method of Sugammadex sodium and intermediate thereof
A technology of sugammadex sodium and its intermediates, which is applied in the field of preparation of sugammadex sodium and its intermediates, can solve the troublesome and dangerous problems of bromine reaction post-processing, and achieve high yield, easy operation, and high production efficiency. The effect of high rate and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
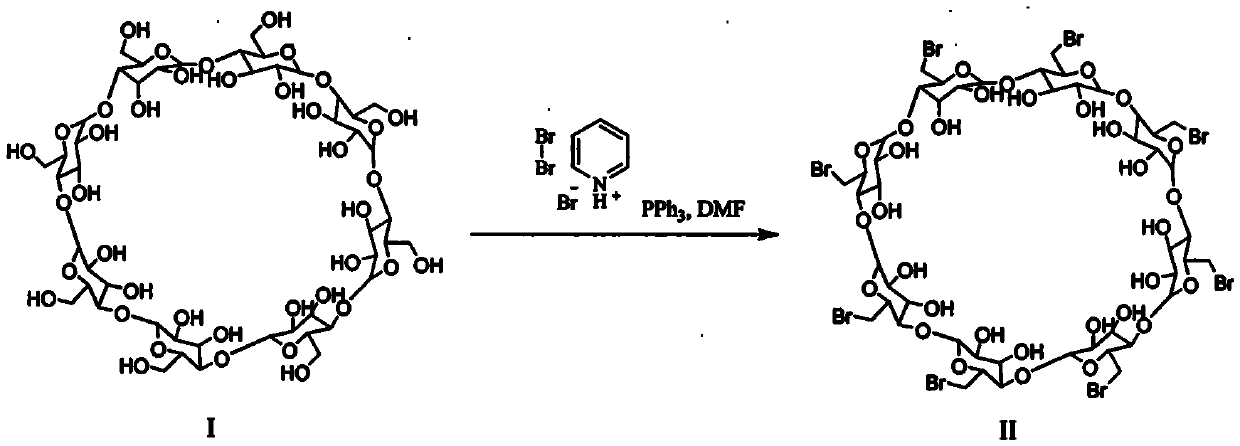
[0044] Preparation of 6-perdeoxy-6-per(2-carboxyethyl)sulfanyl-γ-cyclodextrin (Sugammadex Sodium)
[0045]
[0046] Step 1: Preparation of Perbrominated γ-cyclodextrin (Compound II)
[0047] Add dry N,N-dimethylformamide (10L) into a 50L dry reaction kettle, replace the air with nitrogen, cool with ice water, and add triphenylphosphine (6.48Kg) while stirring. Dissolve pyridinium tribromide (7.89kg) in dry N,N-dimethylformamide (10L), and then add this solution dropwise to the N,N- in dimethylformamide solution. After the drop, the temperature was raised to 20-25°C and stirred for 2 hours, then a suspension of γ-cyclodextrin (2kg) in N,N-dimethylformamide (5L) was added dropwise, and the temperature was controlled at 20-25°C. After dropping, raise the temperature of the reaction solution to 65-70°C, continue the reaction for 16 hours, then cool the reaction solution to 5-10°C, and add 30wt% sodium methoxide methanol solution at this temperature to adjust the pH value to 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com