Method for preparing key intermediate of Tofacitinib
A technology of tofacitinib and intermediates, which is applied in the field of preparation of key intermediates of tofacitinib, can solve the problems of unqualified substances and difficult removal, and achieve low cost, optimized production process and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
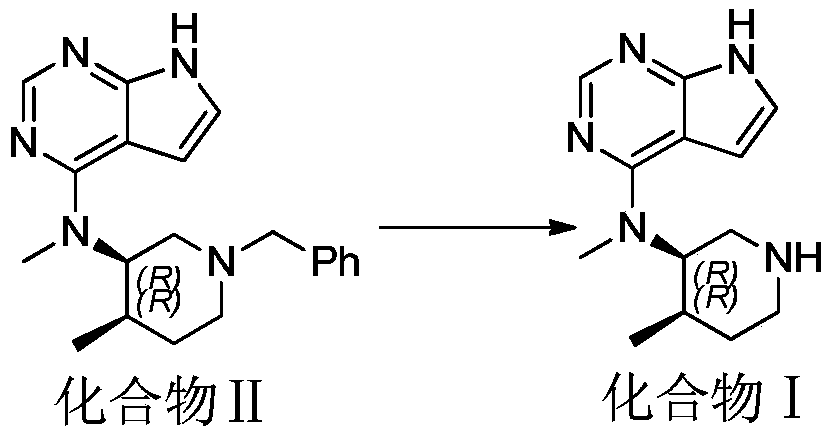
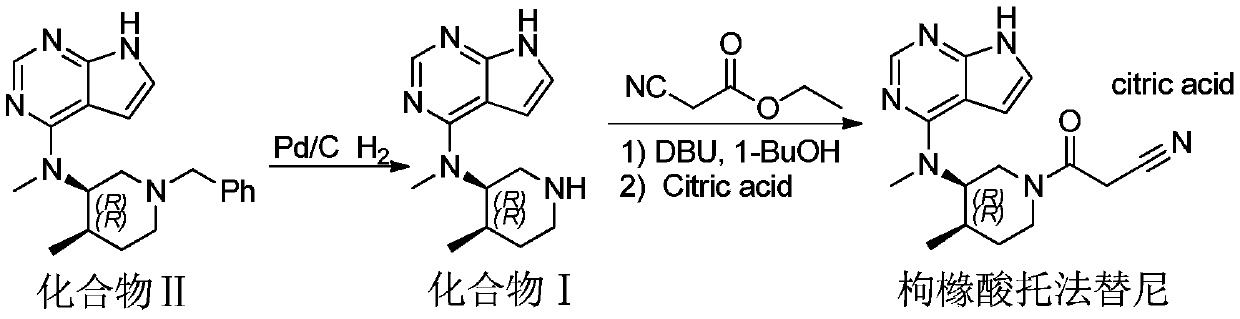
[0012] Embodiment 1, the preparation of compound I
[0013] Add 100g of compound II, 400mL of absolute ethanol, 45g of Pd / C (10%), and 89g of ammonium formate to a 1L reaction flask, and heat in an oil bath to control the temperature of the system at 50°C ± 2°C; TLC detects the reaction progress, After the reaction is complete, cool down to room temperature, filter, and distill the filtrate under reduced pressure to obtain a white flocculent solid. Add 600 ml of dichloromethane to the solid until the solid is completely dissolved. The dichloromethane layer is washed three times with purified water (400 ml), and washed with anhydrous Dry over sodium sulfate and filter. The solvent of the filtrate was concentrated to 1 / 10 of the original volume, and then the concentrated solution was added dropwise to 1000ml of cyclohexane, a white solid was precipitated, and 59.2g of a white solid was obtained by filtration, with a purity of 97.8% and a yield of 81%.
Embodiment 2
[0014] Embodiment 2, the preparation of compound I
[0015] Add 100g of compound II, 600mL of absolute ethanol, 75g of Pd / C (10%), 150g of ammonium formate, and heat in an oil bath to control the temperature of the system at 70°C ± 2°C; After the reaction is complete, cool down to room temperature, filter, and distill the filtrate under reduced pressure to obtain a white flocculent solid. Add 600 ml of dichloromethane to the solid until the solid is completely dissolved. The dichloromethane layer is washed three times with purified water (400 ml), and washed with anhydrous Dry over sodium sulfate and filter. The solvent of the filtrate was concentrated to 1 / 10 of the original volume, and the concentrated solution was added dropwise to 1000 ml of cyclohexane, a white solid was precipitated, and 59.9 g of a white solid was obtained by filtration, with a purity of 98.9% and a yield of 82%.
Embodiment 3
[0016] Embodiment 3, the preparation of compound I
[0017] Add 100g of compound II, 1000mL of absolute ethanol, 150g of Pd / C (10%), ammonium formate 238, and heat in an oil bath to control the temperature of the system at 80°C ± 2°C; TLC detects the reaction progress. After the reaction is complete, cool down to room temperature, filter, and distill the filtrate under reduced pressure to obtain a white flocculent solid. Add 600 ml of dichloromethane to the solid until the solid is completely dissolved. The dichloromethane layer is washed three times with purified water (400 ml), and washed with anhydrous Dry over sodium sulfate and filter. The solvent of the filtrate was concentrated to 1 / 10 of the original volume, and then the concentrated solution was added dropwise to 1000 ml of cyclohexane, a white solid was precipitated, and 60.5 g of a white solid was obtained by filtration, with a purity of 99.1% and a yield of 82.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com