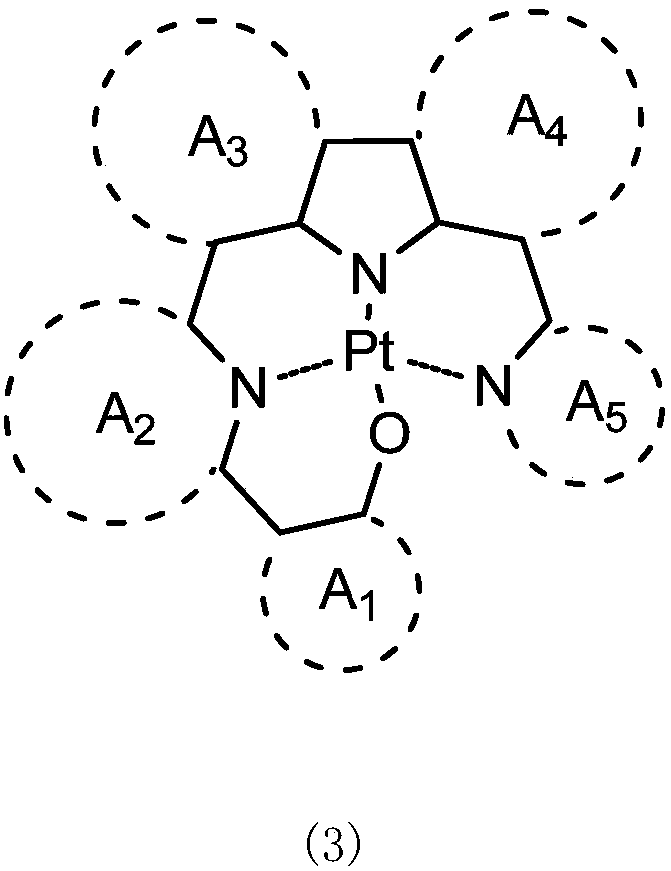
Organic electroluminescent device containing tetradentate platinum (II) complex
A technology of electroluminescent devices and complexes, which is applied in the field of OLED light-emitting devices, and can solve problems such as performance improvement and poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]
[0056] synthetic route:
[0057]
[0058] Synthesis of compound 2: Take 6.50g (20.0mmol) of compound 1, 12.70g (2.5eq., 50.0mmol) of biboronic acid pinacol ester, 5.18g (2.5eq., 50.0mmol) of potassium carbonate and Pd(dppf)Cl 2 292mg (0.02eq., 0.4 mmol), was added to a three-necked flask, evacuated and replaced with nitrogen several times, then injected with 150mL of acetonitrile-dioxane, and heated to 85°C. After reacting for 12 hours under the protection of nitrogen, cool to room temperature, remove the solvent by rotary evaporation, add an appropriate amount of water and ethyl acetate for extraction, collect the organic phase, dry it over anhydrous magnesium sulfate, add an appropriate amount of silica gel, remove the solvent by rotary evaporation, and use n-hexane / Ethyl acetate system column chromatography gave 7.12 g of white solid with a yield of 85% and a purity of 99.0%.
[0059] Synthesis of compound 3: get 11.85g (50.0mmol) compound 2,6-dibromopyrid...
Embodiment 2
[0065]
[0066] synthetic route:
[0067]
[0068] Synthesis of Compound 7: Take 16.72g carbazole (0.10mol) and 655mg anhydrous aluminum trichloride (5mmol) in a three-necked flask, vacuumize and feed nitrogen for replacement several times, then add 27.77g tert-butyl chloride dropwise (3.0eq., 0.30mmol) and 250mL of dry dichloromethane, stirred and reacted for 12hr under the protection of nitrogen, then added an appropriate amount of water for extraction, collected the organic phase, removed the solvent by rotary evaporation, and recrystallized the obtained solid with ethanol to obtain White solid 23.20g, yield 83%, purity 99.5%.
[0069] Synthesis of Compound 8: Take 13.97g (50.0mmol) of Compound 7, dissolve it in 750mL of acetic acid, then drop into 19.98g (2.5eq., 125.0mmol) of liquid bromine, and react in a light-shielding manner. After stirring at room temperature for about 4 hours, remove the solvent by rotary evaporation, then add an appropriate amount of water a...
Embodiment 3
[0076]
[0077] synthetic route:
[0078]
[0079] The synthesis of compound 14: get 7.97g (15.0mmol) compound 9, compound 13 7.63g (1.0eq., 15.0mmol), potassium carbonate 3.45g (1.25eq., 25.0mmol) and Pd (PPh 3 ) 4 347mg (0.02eq., 0.3mmol) was added to a three-necked flask, vacuumed and replaced with nitrogen several times, then injected with 100mL of acetonitrile and 50mL of methanol, and heated to 60°C. After reacting for 12 hours under the protection of nitrogen, cool to room temperature, remove the solvent by rotary evaporation, add an appropriate amount of water and ethyl acetate for extraction, collect the organic phase, dry it over anhydrous magnesium sulfate, add an appropriate amount of silica gel, remove the solvent by rotary evaporation, and use n-hexane / Ethyl acetate system column chromatography gave 7.50 g of a white solid with a yield of 60% and a purity of 99.5%.
[0080] Synthesis of compound 15: take 6.66g (8.0mmol) of compound 14, 1.37g (1.1eq., 8....
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com