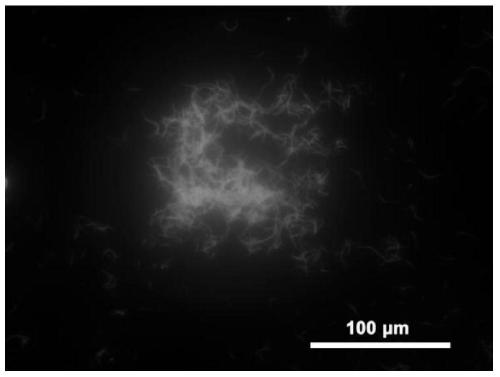
Tetraphenylvinyl bridged polysilsesquioxane and preparing method and application thereof
A technology of polysilsesquioxane and tetraphenylethylene, which is applied in the field of intelligent light-emitting materials, can solve the problems of not having circularly polarized light-emitting properties, and achieve the effects of simple preparation method, convenient operation, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] Two, the preparation of chiral cationic surfactant:
[0061] (1) Dissolve 10-50 mmol of benzyloxy-protected amino acid in 50-200 mL of DMF, then add 10.5-52.5 mmol of HBTU, stir and dissolve, then add 10-50 mmol of redistilled triethylamine. After stirring for half an hour, a DMF solution of 10-50 mmol of alkylamine was slowly added thereto, and the reaction was stirred for 1-10 hours. The reaction liquid was added into deionized water to obtain a flocculent precipitate, which was recrystallized with ethanol to obtain a white solid. It is dissolved in 100-1000 mL of ethanol, and 1-10 g of Pd / C is added, and hydrogen is refluxed for 5-24 hours. The Pd / C was removed by suction filtration, the solvent was removed by rotary evaporation of the filtrate, and the intermediate product A was obtained after recrystallization from petroleum ether.
[0062] (2) Dissolve 10-50 mmol of intermediate product A in 100-1000 mL redistilled CH 2 Cl 2 , add 15-75 mmol redistilled trieth...
Embodiment 1
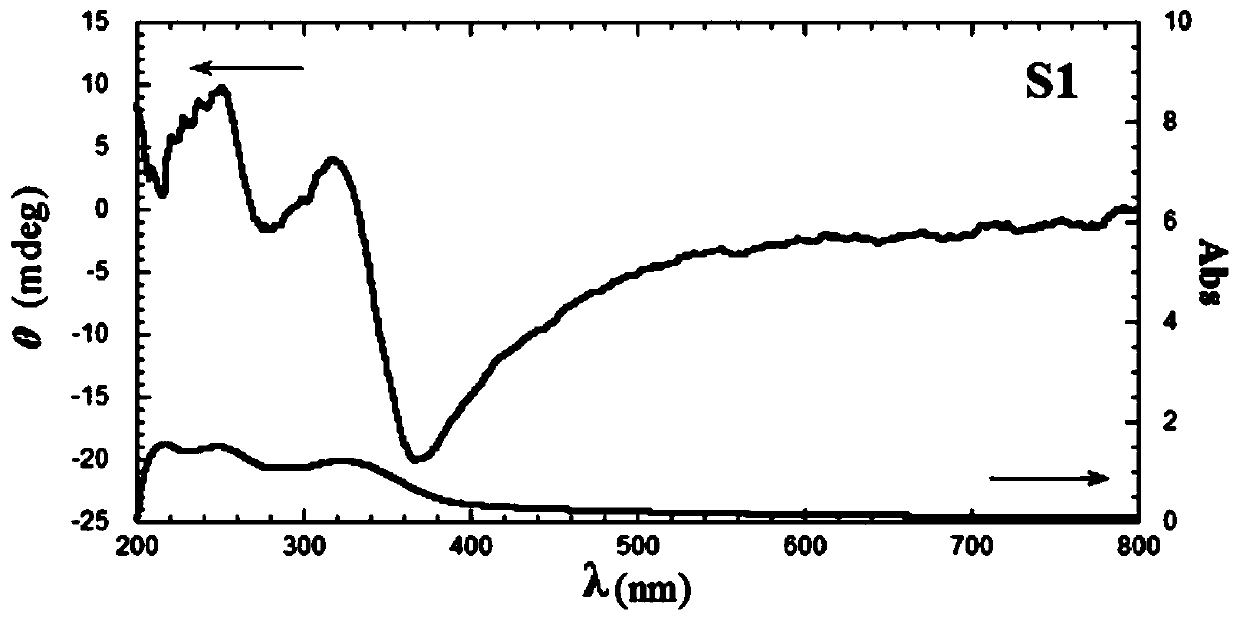
[0068] In this example, tetraphenylvinyl bridged silsesquioxane 1 and cationic surfactant L-18Val11PyBr with the following structures were prepared respectively, and tetraphenylvinyl bridged polysilsesquioxane S1 was further prepared:
[0069] The structural formula of tetraphenylvinyl bridged silsesquioxane 1 is as follows:
[0070]
[0071] The structural formula of the cationic surfactant L-18Val11PyBr is as follows:
[0072]
[0073] The first step, the preparation of tetraphenylvinyl bridged silsesquioxane 1:
[0074] (1) Synthesis of bishalogenated tetraphenylethylene derivatives:
[0075] Weigh 4-bromobenzophenone (13.056g, 50mmol) and zinc powder (13.076g, 200mmol) into a two-necked flask, repeatedly evacuate and protect with nitrogen. 150 mL redistilled tetrahydrofuran (THF) was added to dissolve 4-bromobenzophenone, and then titanium tetrachloride (10.96 mL, 100 mmol) was slowly added dropwise in an ice-water bath. After returning to room temperature, the re...
Embodiment 2
[0088] This example uses tetraphenylvinyl bridged polysilsesquioxane 1 and cationic surfactant D-18Val11PyBr to prepare tetraphenylvinyl bridged polysilsesquioxane S2, wherein tetraphenylvinyl bridged polysilsesquioxane S2 is The structure and preparation method of silsesquioxane 1 are the same as in Example 1.
[0089] (1) The structural formula and preparation method of the cationic surfactant D-18Val11PyBr are as follows:
[0090]
[0091] Take benzyloxy-protected D-valine Z-D-Val (12.564g, 50mmol) and dissolve it in 200mL of N,N-dimethylformamide (DMF) by heating, then add 19.9g (52.5mmol) benzotriazole -N-N-N'-N'-Tetramethyluronium hexafluorophosphate potassium salt (HBTU), after stirring and dissolving, add 5.06 g of redistilled triethylamine (50 mmol). After stirring for half an hour, octadecylamine (13.476 g, 50 mmol) was heated and dissolved in 400 mL of DMF, then slowly added to the reaction vessel, and mechanically stirred for 10 hours. The reaction liquid was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com