Preparation method of chiral molecularly imprinted adsorption-extraction stirring rod
A technology for imprinting adsorption and chiral molecules, which is applied in the field of analytical chemistry and sample pretreatment to achieve the effect of avoiding competitive adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
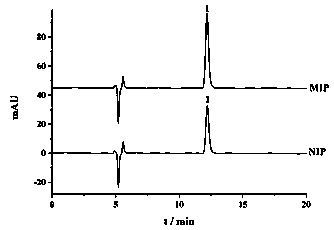
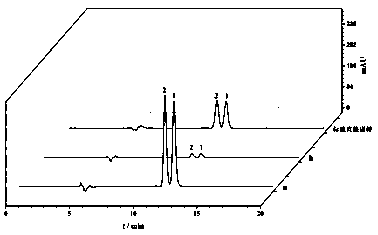
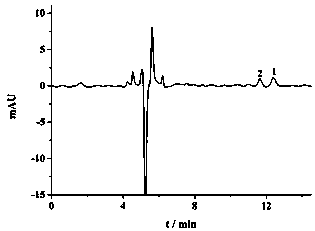
Image
Examples
Embodiment 1
[0030] Example 1: The preparation method of the chiral molecularly imprinted stirring rod described in this example, the specific steps are as follows:
[0031] (1) Clean and dry the iron core and put it into the glass capillary I, then melt and seal the two ends of the glass capillary I to obtain a stirring bar, and prepare the glass capillary II sintered at one end as a jacket;
[0032] (2) Dissolve 1 mmol of the chiral template molecule S-naproxen in 15 mL of methanol-dimethyl sulfoxide (1:1, v / v), and then add the chiral functional monomer L-cysteine Hydrochloride 4 mmol, mixed uniformly, pre-assembled for 10 hours to obtain a pre-assembled solution, then added 30 mmol ethylene glycol dimethacrylate and 20 mg azobisisobutyronitrile to the pre-assembled solution, mixed uniformly, The mixed solution was obtained, and then the mixed solution was ultrasonically deoxygenated and degassed, and then the mixed solution was injected into the glass capillary II using a syringe, and ...
Embodiment 2
[0038] Example 2: The preparation method of the chiral molecularly imprinted stirring rod described in this example, the specific steps are as follows:
[0039] (1) Clean and dry the iron core and put it into the glass capillary I, then melt and seal the two ends of the glass capillary I to obtain a stirring bar, and prepare the glass capillary II sintered at one end as a jacket;
[0040] (2) Dissolve 1 mmol of the chiral template molecule S-naproxen in 15 mL of methanol-dimethyl sulfoxide (1:1, v / v), and then add the chiral functional monomer D-cysteine Hydrochloride 4 mmol, mixed well, pre-assembled for 10 h to obtain a pre-assembled solution, then added 20 mmol ethylene glycol dimethacrylate and 20 mg azobisisobutyronitrile to the pre-assembled solution, mixed well , to obtain a mixed solution, and then ultrasonically deoxygenate and degas the mixed solution, and then use a syringe to inject the mixed solution into the glass capillary II, insert a stirrer, and heat in a wat...
Embodiment 3
[0045] Example 3: The preparation method of the chiral molecularly imprinted stirring rod described in this example, the specific steps are as follows:
[0046] (1) Clean and dry the iron core and put it into the glass capillary I, then melt and seal the two ends of the glass capillary I to obtain a stirring bar, and prepare the glass capillary II sintered at one end as a jacket;
[0047] (2) Dissolve 1 mmol of the chiral template molecule S-naproxen in 15 mL of methanol-dimethylsulfoxide (1:1, v / v), and then add the chiral functional monomer N-acetyl-L - Cysteine 4 mmol, mixed well, pre-assembled for 10 h to obtain a pre-assembled solution, then added 25 mmol ethylene glycol dimethacrylate and 20 mg azobisisobutyronitrile to the pre-assembled solution, mixed homogeneously to obtain a mixed solution, and then ultrasonically deoxygenate and degas the mixed solution, and then use a syringe to inject the mixed solution into the glass capillary II, insert a stirring bar, and hea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com