Application of tanshinone IIA in inhibiting abnormal accumulation of Tau protein
A tanshinone and protein technology, applied in the field of biomedicine, can solve problems such as unsatisfactory drug efficacy, achieve the effects of less toxic and side effects, good curative effect, and inhibiting abnormal aggregation of Tau protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Tanshinone IIA inhibits heparin-induced Tau protein aggregation
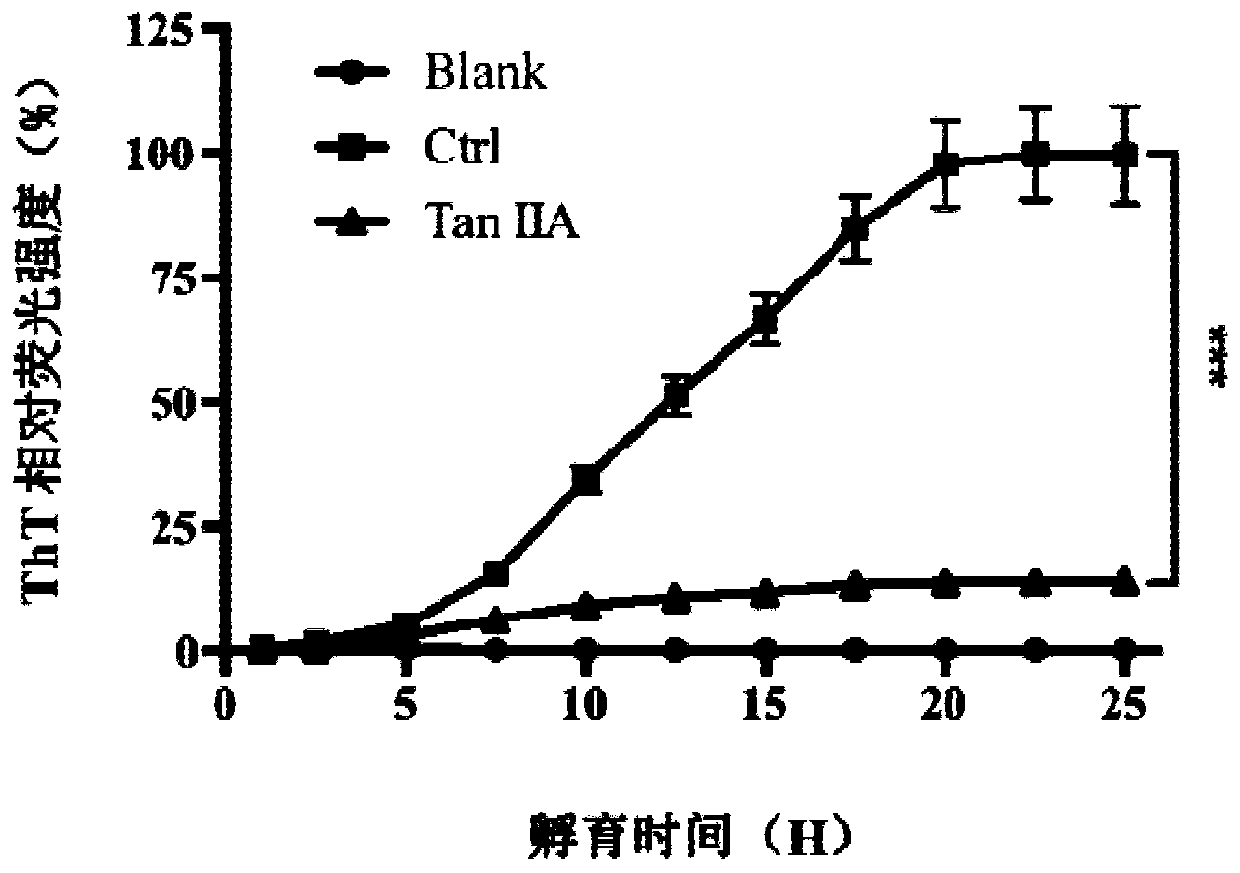
[0045] Tau protein can be induced by heparin sodium in vitro to self-aggregate to form fibers, thereby simulating the pathological process of abnormal aggregation of Tau protein in vivo to produce neurofibrillary tangles. Thioflavin T (ThT) can bind to the β-structure of fibers to emit fluorescence, so the fibrosis process of Tau protein can be tracked through the fluorescence value of ThT. Dissolve Tau protein to 20 μM with Tris-HCl buffer and add to black microplate, then add thioflavin T, heparin sodium and tanshinone IIA (20 μM) Under the excitation light of 485nm, continuous detection was carried out for 25 hours, and the relative fluorescence value was used to make a graph.
[0046] like figure 1 As shown, in vitro, Tau protein aggregates under the induction of heparin sodium, and when tanshinone IIA is added to the system, the aggregation of Tau protein is significantly inhibited.
Embodiment 2
[0047] Example 2: Transmission Electron Microscopy Experiment of Tanshinone IIA Inhibiting Tau Protein Fibrosis
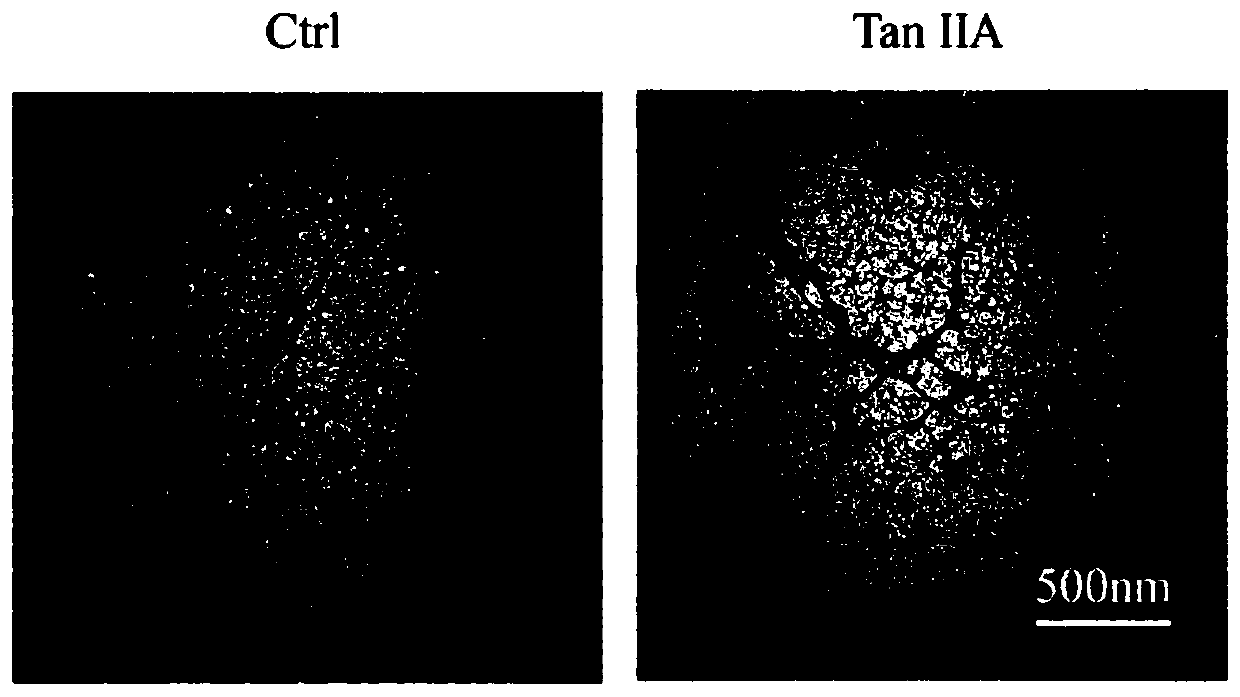
[0048] Methods: The fibrosis of Tau protein samples can be directly observed by transmission electron microscopy (TEM), which can further verify the results of ThT fluorescence experiments. Take 5 μL of each sample in the Tau protein ThT experiment, drop it on a 230-mesh copper grid, then absorb the excess sample with filter paper, drop 5 μL of uranyl acetate on each after drying, absorb the excess liquid after staining for 1 minute, dry and place in In a transmission electron microscope (NIPPON TEKNO, JEM-1230), observe and take pictures. Result: results like figure 2 As shown, dense Tau protein fibers were observed under the transmission electron microscope in the control group, but after the addition of tanshinone IIA, the Tau protein fibers were sparse and fractured, indicating that tanshinone IIA can effectively hinder the formation of Tau protein fibers.
Embodiment 3
[0049] Example 3: Molecular docking of Tanshinone IIA and Tau protein
[0050] The Autodock vina 1.1.2 program was used for molecular docking to study the binding mode between tanshinone IIA and Tau protein. The core structure of the Tau protein fiber (PDB number 5O3L) was downloaded from the RCSB protein database (http: / / www.rcsb.org / pdb / home / home.do). The 2D results of Tanshinone IIA were drawn by ChemBioDraw Ultra 12.0 software, and the 3D structure of the molecule was optimized by ChemBio3D Ultra 12.0. Use AutoDockTools 1.5.6 to process and dock the protein after hydrogenation. The coordinates of the center point of the docking port are (215.348, 133.426, 155.072), size_x, size_y, and size_z are 15, 15, 15, and 15, respectively, and the global search exhaustiveness (exhaustiveness ) was set to 20, other parameters were set to default values, the best docking method was selected by docking scoring, and visual analysis was performed by PyMoL1.7.6 software.
[0051] like ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com