Carbazochrome sodium sulfonate and content assay method for semicarbazide hydrochloride in carbazochrome sodium sulfonate preparation
A technology of semicarbazide hydrochloride and carbosulfonate, which is applied in the field of drug analysis, can solve the problems of affecting detection efficiency and accuracy, affecting the accuracy of results, and not applicable to carbosulfonodium, etc., achieving good application prospects, convenient operation, effect of satisfying novelty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
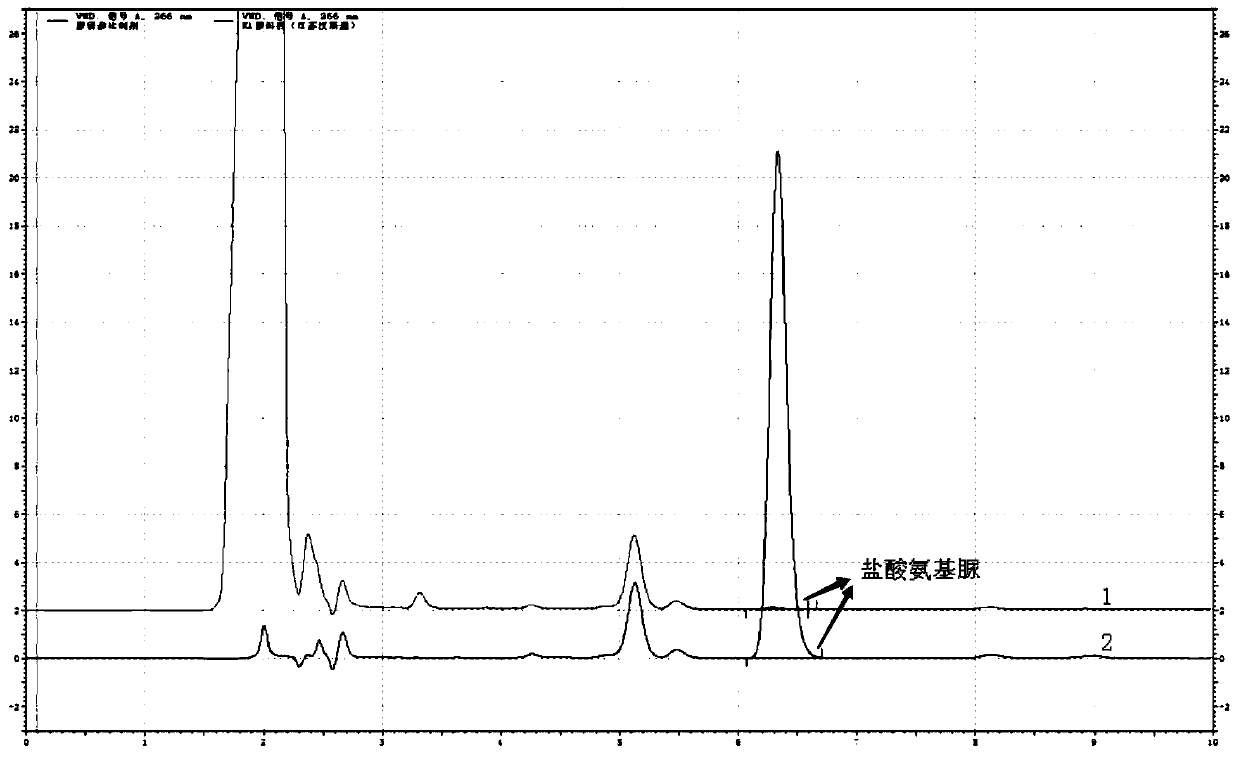
[0045] The determination of the content of semicarbazide hydrochloride in embodiment 1 carbosulfonium sodium
[0046] Sample to be tested Sodium carbosulfonate
[0047] (1) Sample preparation and derivatization reaction
[0048] a. Blank solution: Take 4ml of methanol and appropriate amount of water, put it in a 10ml volumetric flask, use hydrochloric acid solution (3→1000) to adjust the pH to between 3.5 and 5.5, shake well, and then add 1.0ml of 2-naphthaldehyde (NCA) Solution, and dilute to volume with water, shake well; react this solution at room temperature for 2 hours.
[0049] b. Reference substance solution: take 10mg of semicarbazide hydrochloride, weigh it accurately, put it in a 100ml volumetric flask, add an appropriate amount of water to dissolve it, and dilute to the mark, shake well; accurately measure 5ml to a 50ml volumetric flask, add water to dilute to the mark Shake well, as the reference substance stock solution; accurately measure 1.0ml, put it in a 10...
Embodiment 2
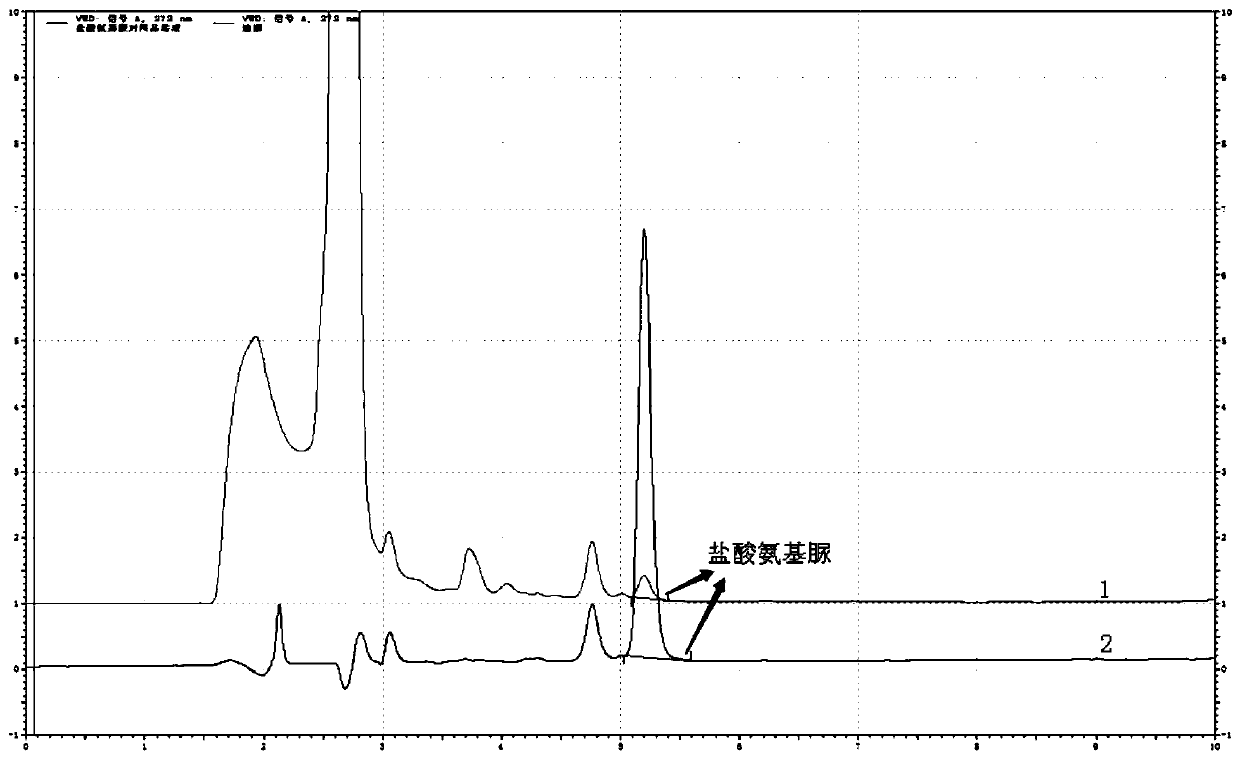
[0066] The determination of the content of semicarbazide hydrochloride in embodiment 2 carbosulfonium sodium injection
[0067] The specification of the sample to be tested is 20ml: 100mg
[0068] (1) Preparation and derivation process of the test product and reference product
[0069] a. Blank solution: Take 4ml of methanol and appropriate amount of water, put it in a 10ml volumetric flask, use hydrochloric acid solution (3→1000) to adjust the pH to 3.5~5.5, shake well, then add 1.0ml of NCA solution, and dilute with water, shake well ; The solution was reacted at room temperature for 2 hours.
[0070] b. Reference substance solution: take 10mg of semicarbazide hydrochloride, weigh it accurately, put it in a 100ml volumetric flask, add an appropriate amount of water to dissolve it, and dilute to the mark, shake well; accurately measure 5ml to a 50ml volumetric flask, add water to dilute to the mark Shake well, as the reference substance stock solution. Precisely measure 1....
Embodiment 3
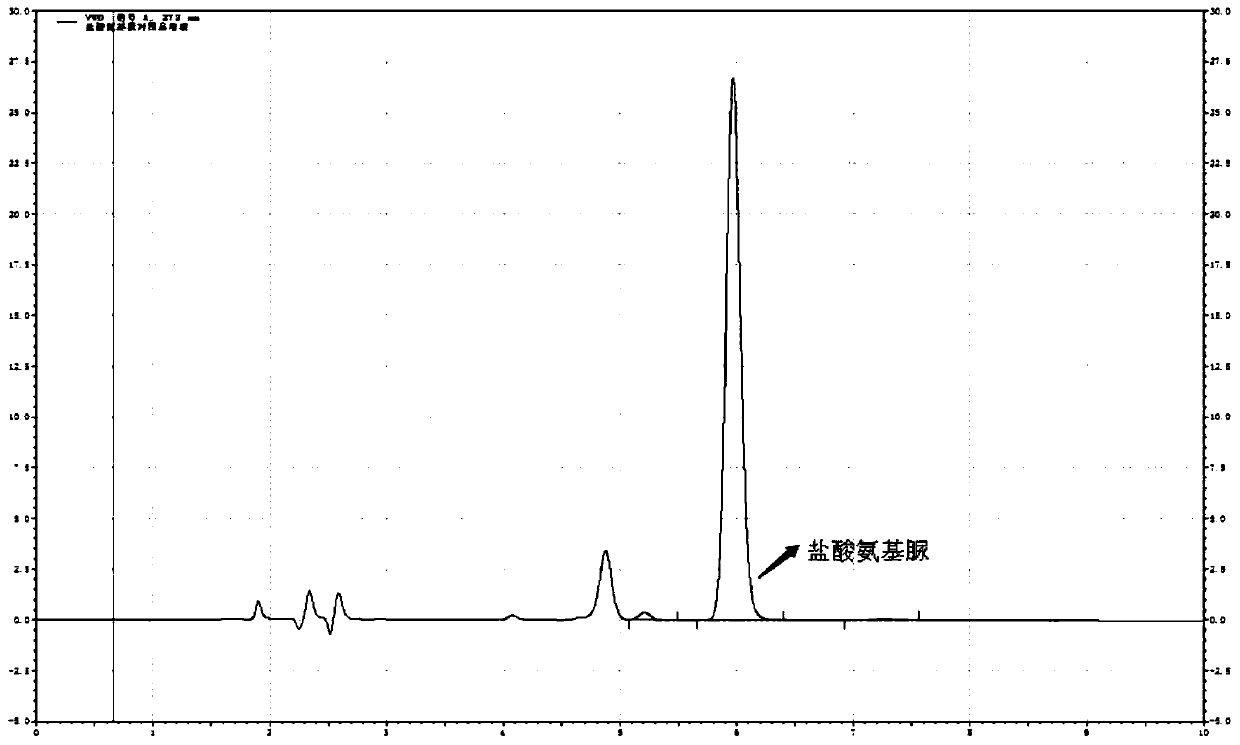
[0087] The determination of the content of semicarbazide hydrochloride in embodiment 3 carbosulfonium sodium chloride injection
[0088] The sample to be tested is carbosulfonium sodium chloride injection. The specification is 100ml: 80mg
[0089] (1) Preparation and derivation process of the test product and reference product
[0090] a. Blank solution: Take 4ml of methanol and appropriate amount of water, put it in a 10ml volumetric flask, use hydrochloric acid solution (3→1000) to adjust the pH to 3.5~5.5, shake well, then add 1.0ml of NCA solution, and dilute with water, shake well ; The solution was reacted at room temperature for 2 hours.
[0091] b. Reference substance solution: take 10mg of semicarbazide hydrochloride, weigh it accurately, put it in a 100ml volumetric flask, add an appropriate amount of water to dissolve it, and dilute to the mark, shake well; accurately measure 1ml to a 50ml volumetric flask, add water to dilute to the mark Shake well, as the refere...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com