Method for synthesizing 1,9-decadiene
A synthesis method and technology of decadiene are applied in the field of preparation of organic compound 1,9-decadiene, can solve the problems of expensive catalyst, complex reaction conditions, large amount of catalyst, etc., and achieve good industrial application value and product purity The effect of high and high utilization rate of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, a kind of synthetic method of 1,9-decadiene, carries out following steps successively:
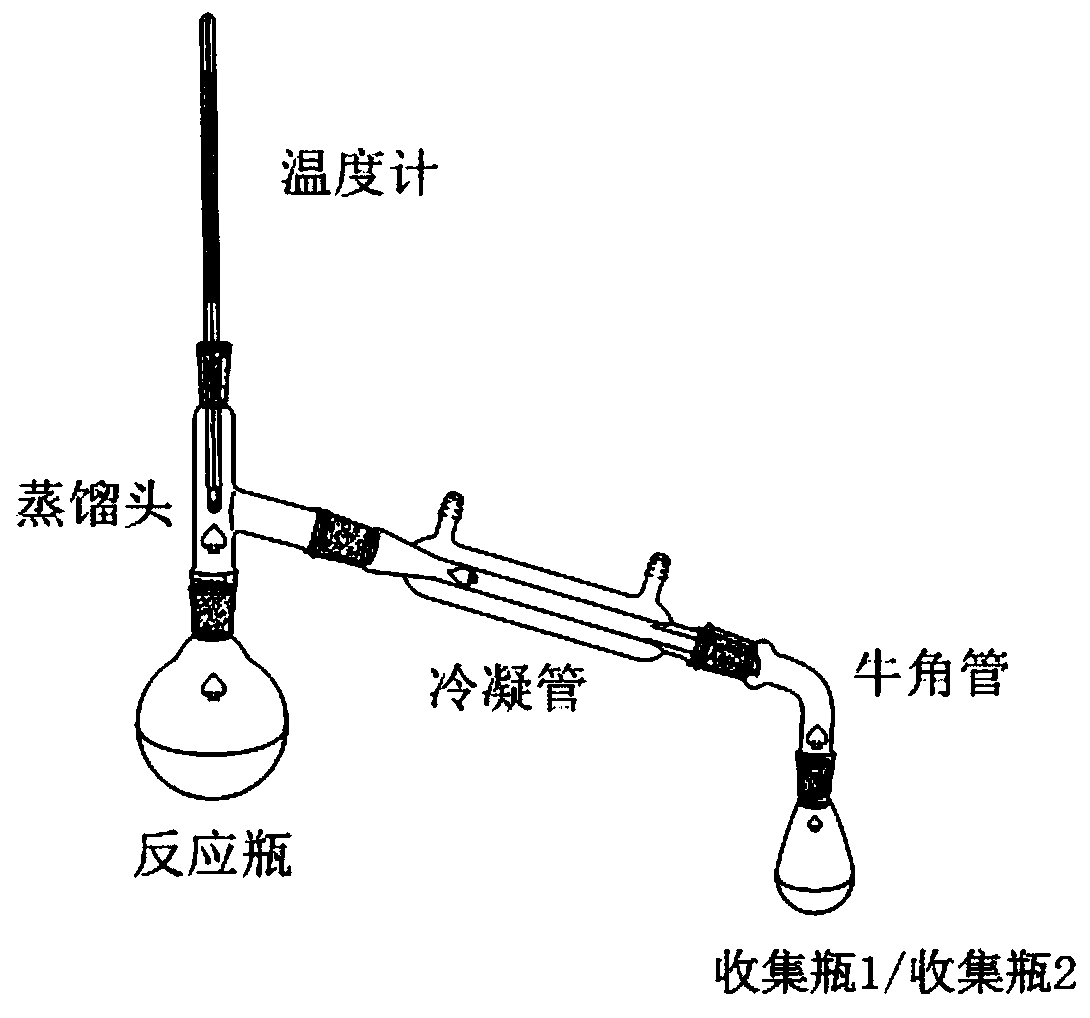
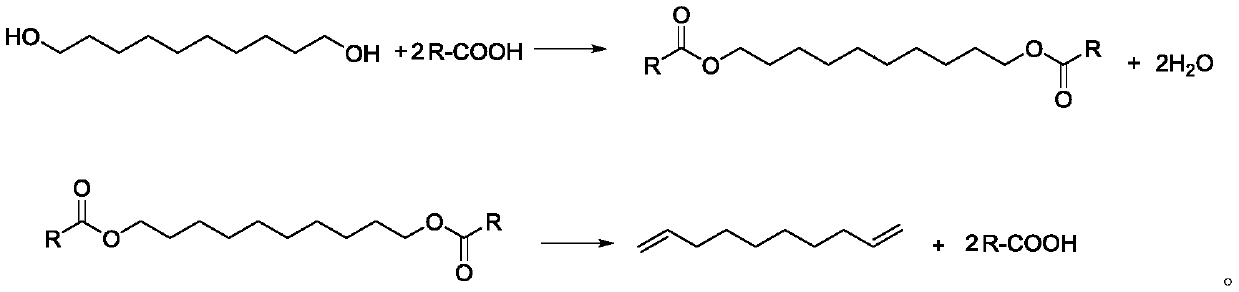
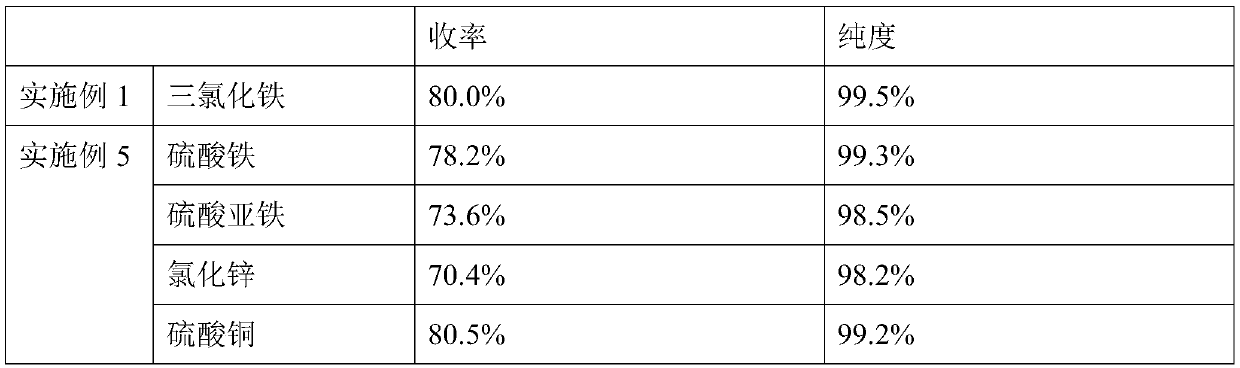
[0037] (1), after mixing 34.8g (0.2mol) 1,10-decanediol, 48.0g (0.8mol, 4eq) glacial acetic acid and 3.5g ferric chloride, place it in a 250mL reaction flask as a reaction solution, and heat up to 120°C, carry out esterification reaction and distill water, collect water through collection bottle 1;
[0038] (2) After 3 hours of esterification reaction, no more water will come out, continue to heat up to 350°C, steam the mixture of 1,9-decadiene and acetic acid, continue to discharge for 8 hours (that is, the reaction time is 8 hours), and pass through the collection bottle 2. Collect the mixed solution of 1,9-decadiene and acetic acid;
[0039] (3), remove the acetic acid (117°C) from the discharge (1,9-decadiene and acetic acid mixture) obtained in step (2) under normal pressure, then carry out vacuum distillation, collect 68±0.5°C (20Torr ) fraction to obtain 22.1g...
Embodiment 2
[0043] Embodiment 2, a kind of synthetic method of 1,9-decadiene, carry out following steps successively:
[0044] 1) Mix 34.8g (0.2mol) of 1,10-decanediol, 59.2g (0.8mol, 4eq) of propionic acid and 3.5g of copper sulfate and place it in a 250mL reaction flask as a reaction solution, heat up to 140°C, Carry out esterification reaction and steam out water, collect water by collecting bottle 1;
[0045] (2) After 4 hours of esterification until no more water comes out, continue to heat up to 340°C, steam the mixture of 1,9-decadiene and propionic acid, continue discharging for 8 hours, and collect 1,9- A mixture of decadiene and acetic acid;
[0046] (3) Distill propionic acid (141°C) from the output obtained in step (2) under normal pressure, and then conduct vacuum distillation to collect fractions at 68±0.5°C (20 Torr) to obtain 1,9-decadiene product 21.5 g (purity is 99.2%), yield is 78%;
[0047] (4), cyclic reaction:
[0048] After the step (2) is completed, the solid-...
Embodiment 3
[0050]Embodiment 3, a kind of synthetic method of 1,9-decadiene, carries out following steps successively:
[0051] (1), after mixing 34.8g (0.2mol) 1,10-decanediol, 73.2g (0.6mol, 3eq) benzoic acid and 3.5g ferric chloride, place it in a 250mL reaction flask as a reaction solution, and heat up to 180°C, carry out esterification reaction and distill out water, and collect water through collection bottle 1;
[0052] (2) After 3 hours of esterification until no more water comes out, continue to heat up to 330°C, steam the mixture of 1,9-decadiene and benzoic acid, continue to discharge for 8 hours, and collect 1,9- A mixture of decadiene and acetic acid;
[0053] (3), the discharge obtained in step (2) is subjected to vacuum distillation, and the fraction at 68±0.5°C (20 Torr) is collected, and the still liquid is a benzoic acid solution to obtain 22.6g of 1,9-decadiene product (purity is 99.3 %), the yield is 82%;
[0054] (4), cyclic reaction:
[0055] After the step (2) i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com