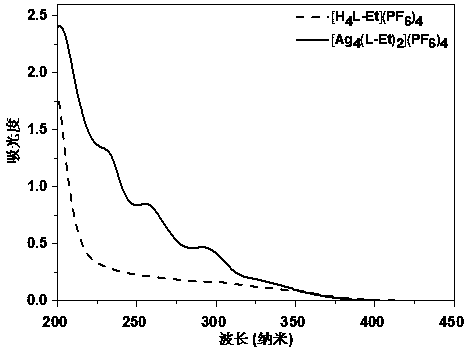
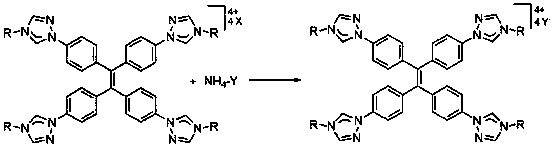
Cage-like metal compound with strong fluorescence emission and synthesis method thereof
A compound and complex technology, applied in the field of strong fluorescence emission caged metal compounds and their synthesis, to achieve the effects of high yield, good luminescence performance and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: tetradentate triazolium salt ligand [H 4 L-Me](PF 6 ) 4 preparation of
[0047] The preparation of the tetraphenylethylene ligand of tetratriazole: 1 mmol of tetraiodotetraphenylethene, 8 mmol of 1,2,4-triazole, 8 mmol of CS in sequence under nitrogen atmosphere 2 CO 3 Add 0.8mmol of CuI to a 100ml schlenk reaction tube, add 40mL of DMF, and react in the dark at 120°C for 48 hours. After the reaction, cool to room temperature, spin dry the DMF, add 500ml of water, filter to obtain a filter residue, and use Extracted with DMF and spin-dried to obtain 0.52 g of white powdery solid with a yield of 86%. . 1 H NMR (400 MHz, DMSO- d 6 ): δ = 9.25 (s, 4H, H1), 8.20 (s, 4H, H2), 7.73 (d, 8H, 3 J = 8.0 Hz,H4), 7.25 ppm (d, 8H, 3 J = 8.0 Hz, H5). 13 C{ 1 H) NMR (400 MHz, DMSO- d6 ): δ =152.4 (C2), 142.2 (C1), 141.9 (C3), 139.7 (C7), 135.3 (C6), 132.2 (C5), 119.0ppm (C4).
[0048] Under anhydrous and oxygen-free operating conditions, tetratriazo...
Embodiment 2
[0051] Embodiment 2: tetradentate triazolium salt ligand [H 4 L-Et](PF 6 ) 4 preparation of
[0052] Under anhydrous and oxygen-free operating conditions, tetratriazole tetraphenylethylene (1.0 mmol), bromoethane (4.0 mmol), (the ratio of the two substances is 1:4), DMF solution (20 mL) Place in a 100 ml Schlenk bottle, reflux at 110°C for 12-24 hours, cool to room temperature, wash with ethyl acetate, filter and drain, put the product in a flask and dissolve it in 150 mL of methanol, add 4.5 mmol of ammonium hexafluorophosphate, and store at room temperature The reaction was stirred for 2 h, during which a large amount of white precipitate was produced, which was filtered and dried, and the product was washed with ether and dried in vacuo. 0.97 g of colorless solid powder was obtained, with a yield of 75%. 1 H NMR (400 MHz, DMSO- d 6 ): δ = 10.82(s, 4H, H1), 9.43 (s, 4H, H2), 7.80 (d, 3 J = 8.0 Hz, 8H, H4), 7.43 (d, 3 J =8.0 Hz, 8H, H5), 4.33-4.30 (m, 8H, H8), ...
Embodiment 3
[0055] Embodiment 3: Silver carbene complex [Ag 4 (L-Me) 2 ](PF 6 ) 4 preparation of
[0056] Under anhydrous and oxygen-free operating conditions, the ligand [H 4 L-Me](PF 6 ) 4 0.05 mmol was dissolved in 20 mL of acetonitrile, then 0.1 mmol Ag was added to the solution 2 O powder, stirred and reacted at 55 °C for 48 h in the dark. After the reaction was completed, cool to room temperature, absorb the supernatant after settling, concentrate the supernatant to 3 mL in the dark, add a large amount of diethyl ether, precipitate a white solid, collect the solid by filtration, wash with diethyl ether, and drain to obtain 2.02 g of a colorless solid powder. Yield 87%. 1 H NMR (400 MHz, CD 3 CN) : δ = 8.51 (s, 8H, H2), 7.73 (br, 16H, H4), 7.29 (d, 3 J = 7.9 Hz, 16H, H5), 4.02ppm (s, 24H, H8). 13 C{ 1 H) NMR (100 MHz, CD 3 CN): δ = 181.7 (C1), 146.0 (C2), 143.7 (C3), 139.4 (C6), 133.0 (C5), 123.0 (C7), 122.9 (C4), 37.0 ppm (C8). MALDI-TOF-MS: m / z = 1017.0507 (c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com