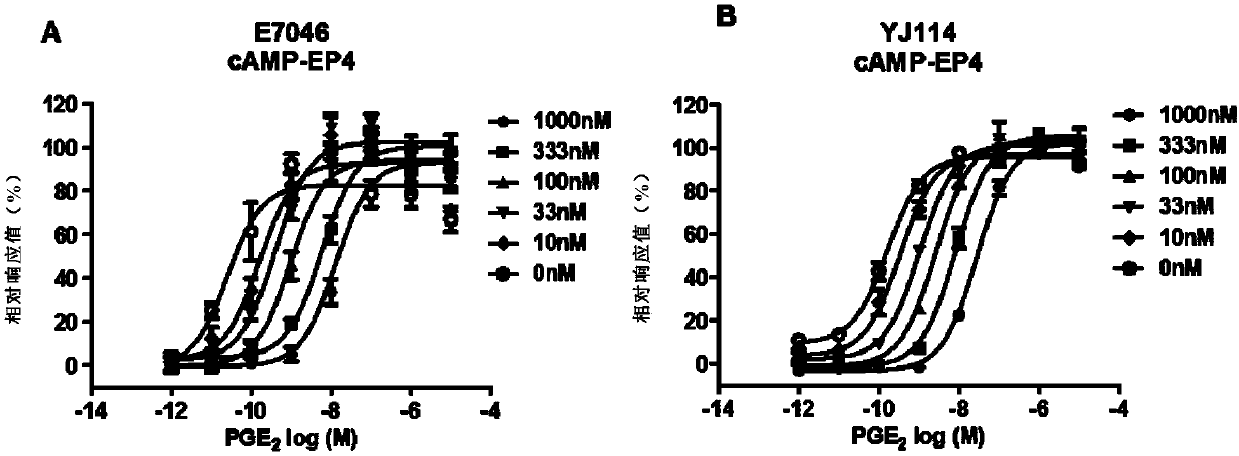
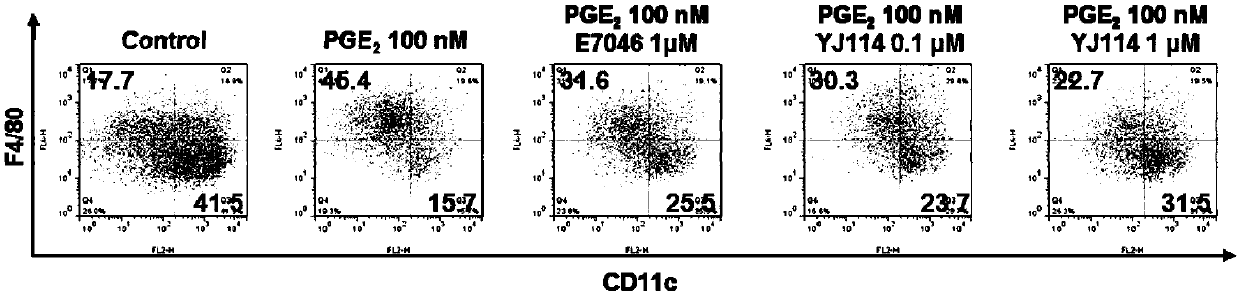
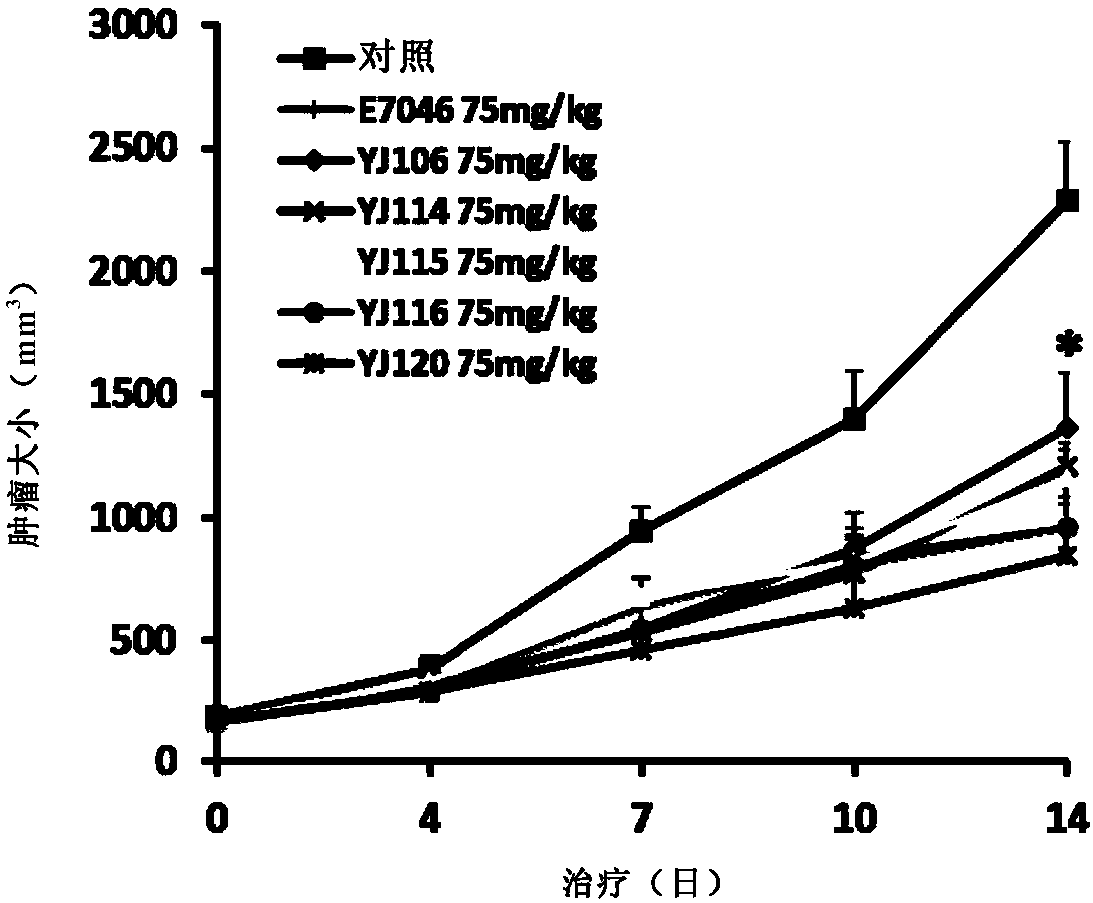
Thieno-ring compound and synthesis method and application thereof
A compound and hydrate technology, applied in the field of biomedicine, can solve problems such as deficiency, increased expression of synthetase COX2, and increased PGE2 level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0182] The preparation of formula I compound
[0183] Exemplary formula I compound preparation process is shown in following table 1:
[0184] Table 1
[0185]
[0186]
[0187]
[0188]
[0189] Pharmaceutical composition and its use
[0190] Another aspect of the present invention provides a pharmaceutical composition, which contains a therapeutically effective amount of the compound selected from the above general formula (I), its pharmaceutically acceptable salts, enantiomers, diastereoisomers or racemate, and optionally, one or more pharmaceutically acceptable carriers, excipients, adjuvants, excipients and / or diluents. The excipients are, for example, flavoring agents, flavoring agents, sweetening agents, and the like.
[0191] The pharmaceutical composition provided by the present invention preferably contains active ingredients in a weight ratio of 1-99%, and its preferred ratio is that the compound of general formula I accounts for 65wt%-99wt% of the to...
Embodiment 1-1
[0197] Example 1-1 , (S)-4-(1-(2-((4-fluorophenyl)ethynyl)-5,7-dihydro-4H-thieno[2,3-c]pyran-3-carboxamide ) ethyl) the preparation of benzoic acid (YJ101)
[0198]
[0199] Tetrahydro-4H-pyran-4-one (2.00g, 20.0mmol), ethyl cyanoacetate (2.50g, 22.0mmol) and sulfur (704mg, 22.0mmol) were dissolved in 30.0mL ethanol, and then added to the solution Morpholine (1.74 g, 20.0 mmol) was added to it, and stirred overnight at 50°C. Use TLC to detect the reaction, after the reaction is completed, the reaction solution is extracted with ethyl acetate and water, the upper organic phase is taken and evaporated to dryness, and purified by column chromatography to obtain a light yellow solid, namely 2-amino-5,7-dihydro -4H-Thieno[2,3-c]pyran-3-carboxylic acid ethyl ester (4.38 g, yield 96%). Dissolve ethyl 2-amino-5,7-dihydro-4H-thieno[2,3-c]pyran-3-carboxylate (450 mg, 2.0 mmol) in 1.5M HCl (10.0 mL) at room temperature Stir for 20 min, then lower the temperature to 0 °C under ice...
Embodiment 1-2
[0200] Example 1-2 , (S)-4-(1-(2-((4-fluorophenyl)ethynyl)-5,6-dihydro-4H-cyclopentadiene[b]thiophene-3-carboxamide)ethyl Base) Preparation of Benzoic Acid (YJ102)
[0201]
[0202] Using the same reaction route as for the preparation of compound YJ101, replacing tetrahydro-4H-pyran-4-one with cyclopentanone, compound YJ102 was finally obtained (the yield of the last step reaction was 62%). 1 H NMR (500MHz, DMSO) δ8.59(d, J=7.5Hz, 1H), 7.81(d, J=7.6Hz, 2H), 7.52(d, J=7.7Hz, 2H), 7.45-7.43(m ,2H),7.23(t,J=8.4,8.8Hz,2H),5.18–5.13(m,1H),1.80(m,6H),1.45(d,J=6.8Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com