recombinant human corticotropin (ACTH) precursor for improving serum glucocorticoid levels and preparation method
A technology of glucocorticoid and gene recombination, which is applied in the direction of adrenocorticotropic hormone, chemical instruments and methods, recombinant DNA technology, etc., can solve the problems of high production cost, transmission of animal viruses and mycoplasma, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
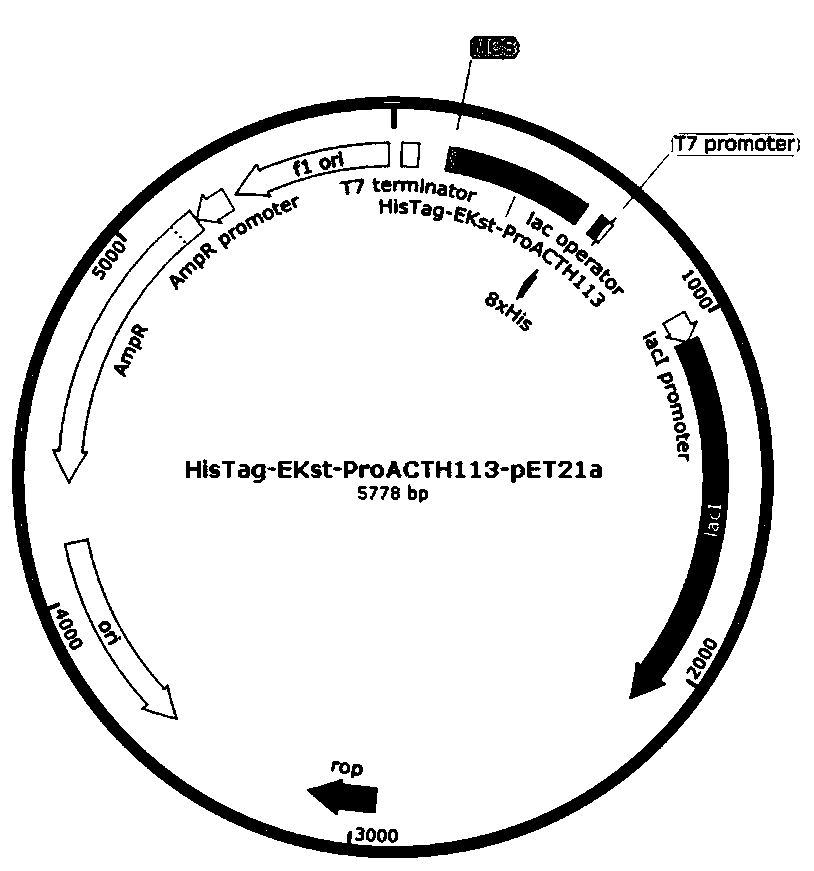
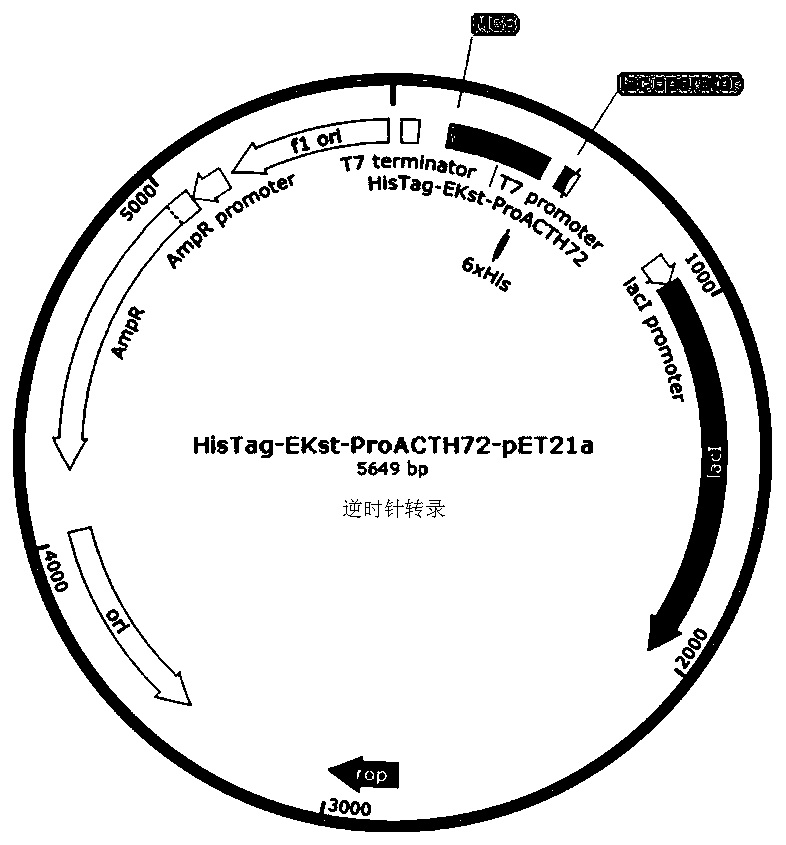
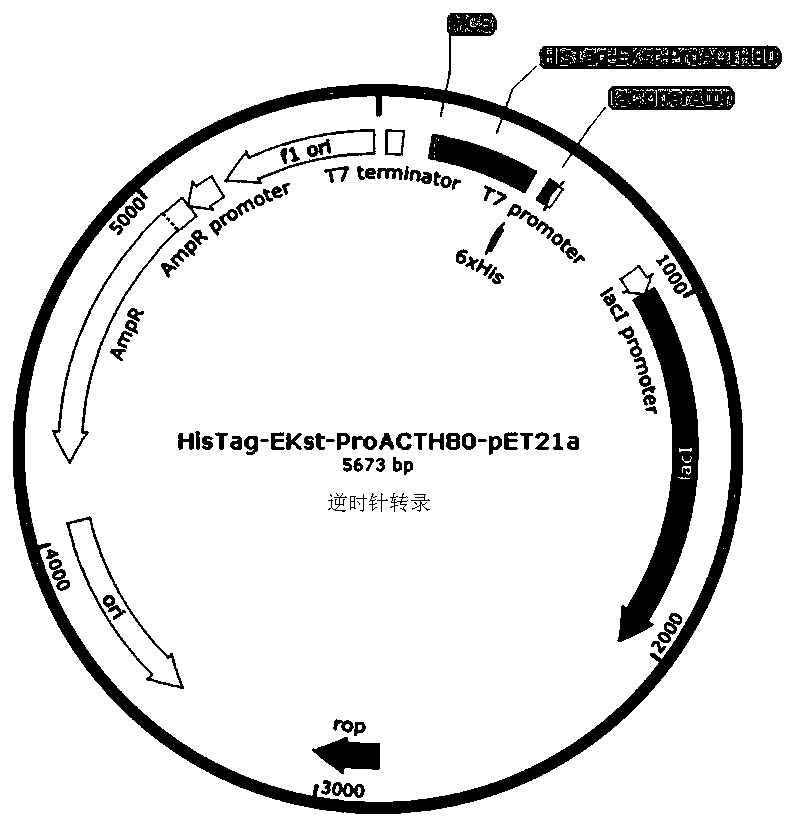
[0056] Specific embodiment 1: In this embodiment, expression, separation and purification of gene recombinant ProACTH113, ProACTH72, ProACTH80 and ProACTH141 fusion proteins are carried out according to the following steps:
[0057] 1. The ProACTH141 CDS sequence was optimally designed according to the codon preference in the prokaryotic expression system, so as to be suitable for high-efficiency expression in E. coli expression strain BL21-DE3. Using NdeI and SalI as the restriction sites at the upstream and downstream ends of the CDS sequence of the fusion expression protein, the prokaryotic expression sequence NdeI-HisTag-EKst-ProACTH141-SalI of the fusion protein with His tag and enterokinase restriction site was constructed. This sequence will serve as a template for PCR amplification of ProACTH113, ProACTH72 and ProACTH80 sequences. Specific steps are as follows:
[0058] Primer design: Using the above optimized NdeI-HisTag-EKst-ProACTH141-SalI sequence as a template, d...
specific Embodiment approach 2
[0068] Embodiment 2: This embodiment differs from Embodiment 1 in that pET21a plasmid is not used, but pET30a is used as the expression vector.
specific Embodiment approach 3
[0069] Embodiment 3: This embodiment is different from Embodiment 1 or 2 in that pET30a or pET21a plasmids are not used, but pET28a is used as the expression vector.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com