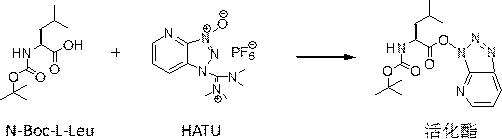
Production method of N-tert-butoxycarbonyl-L-leucyl-L-methyl phenylalanine
A technology of phenylalanine methyl ester and tert-butoxycarbonyl, applied in the field of continuous production of N-tert-butoxycarbonyl-L-leucyl-L-phenylalanine methyl ester, can solve the problem of long reaction time, Low yield, inconvenient storage of activated esters and need to be used as soon as possible to achieve the effects of shortened reaction time, high material utilization, easy to carry and mobile production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
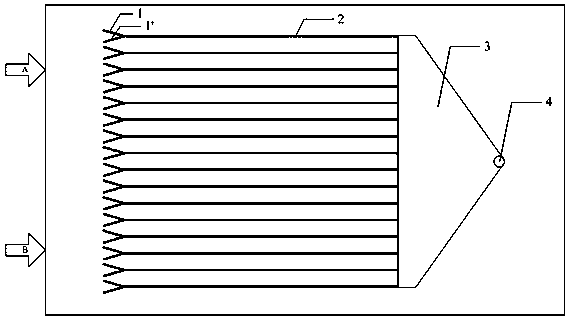
Image
Examples
Embodiment 1
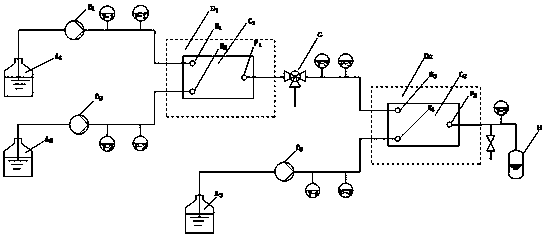
[0045] Two Series II advection pumps (Scientific Systems, Inc.) were used to control the output of HATU solution and N-Boc-L-Leu solution, so that both HATU and N-Boc-L-Leu were simultaneously pumped into microreactor 1 at 1ml / min , the residence time of the fluid in the microreactor 1 is 0.5min, the molar ratio of HATU to N-Boc-L-Leu is 1.05:1, the temperature of the microreactor 1 is controlled by a constant temperature water bath to be 50°C, and the three-way valve G from The concentration of activated ester produced by bypass test was 1.575 mol / L. The activated ester flowing out of the outlet of microreactor 1 and the L-Phe-OMe HCl controlled by the Series II advection pump (Scientific Systems, Inc.) at an output flow rate of 1ml / min were simultaneously pumped into microreactor 2, and the microreactor The residence time of the fluid in 2 is 2.5min, the molar ratio of N-Boc-L-Leu to L-Phe-OMe HCl is 1.05:1, and the temperature of microreactor 2 is controlled by a constant t...
Embodiment 2
[0048]The first microreactor and the second microreactor reaction temperature are 60 ℃, all the other conditions are identical with embodiment 1. Through the three-way valve G from the bypass test to produce the concentration of activated ester is 1.575 mol / L. Implementation results: The concentration of activated esters produced by the bypass test through the three-way valve G is 1.575 mol / L. In collection bottle H: L-Phe-OMe·HCl was completely converted, and the yield of the product N-tert-butoxycarbonyl-L-leucyl-L-phenylalanine methyl ester was 99.2%.
Embodiment 3
[0050] The first microreactor and the second microreactor reaction temperature are 40 ℃, all the other conditions are identical with embodiment 1. Implementation results: The concentration of activated esters produced through the three-way valve G from the bypass inspection is 1.55 mol / L. In collection bottle H: L-Phe-OMe·HCl was completely converted, and the yield of the product N-tert-butoxycarbonyl-L-leucyl-L-phenylalanine methyl ester was 98.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com