Application of neuraminic acid and neuraminidase inhibitor in chronic heart failure
A technology of chronic heart failure and neuraminic acid, applied in the direction of organic active ingredients, cardiovascular system diseases, medical preparations containing active ingredients, etc., can solve problems such as difficulties in diagnosis and treatment of chronic heart failure, insufficient risk assessment, etc., to achieve The effect of improving efficiency and accuracy, rapid detection means, and huge application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
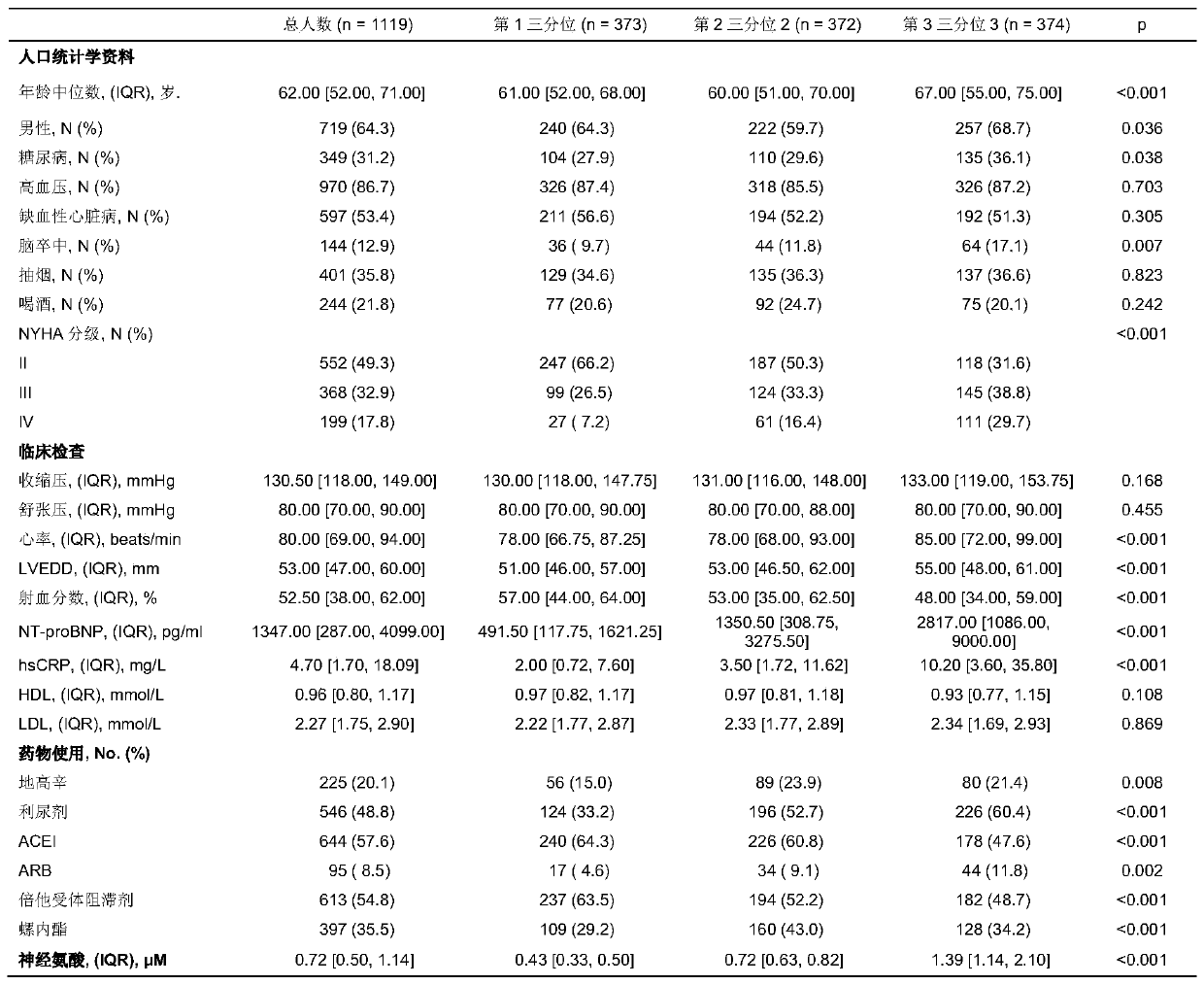
[0036] High plasma Neu5Ac levels are associated with severe chronic heart failure
[0037] Study population and study design: From January 2008 to March 2017, two medical centers (Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology in Wuhan City, Hubei Province, and the Second Affiliated Hospital of Hebei Medical University in Shijiazhuang, Hebei Province) were continuously included. Hospitalized patients with chronic heart failure. Inclusion criteria include: age greater than 18 years old, heart failure NYHA classification II-IV. The diagnosis of heart failure is determined on the basis of physical examination, laboratory examination, and echocardiography according to the diagnostic criteria and steps of ACC / AHA. Exclusion criteria include: heart failure caused by severe valvular disease; patients with life-threatening complications such as severe liver dysfunction and renal dysfunction; survival time of patients with maligna...
Embodiment 2
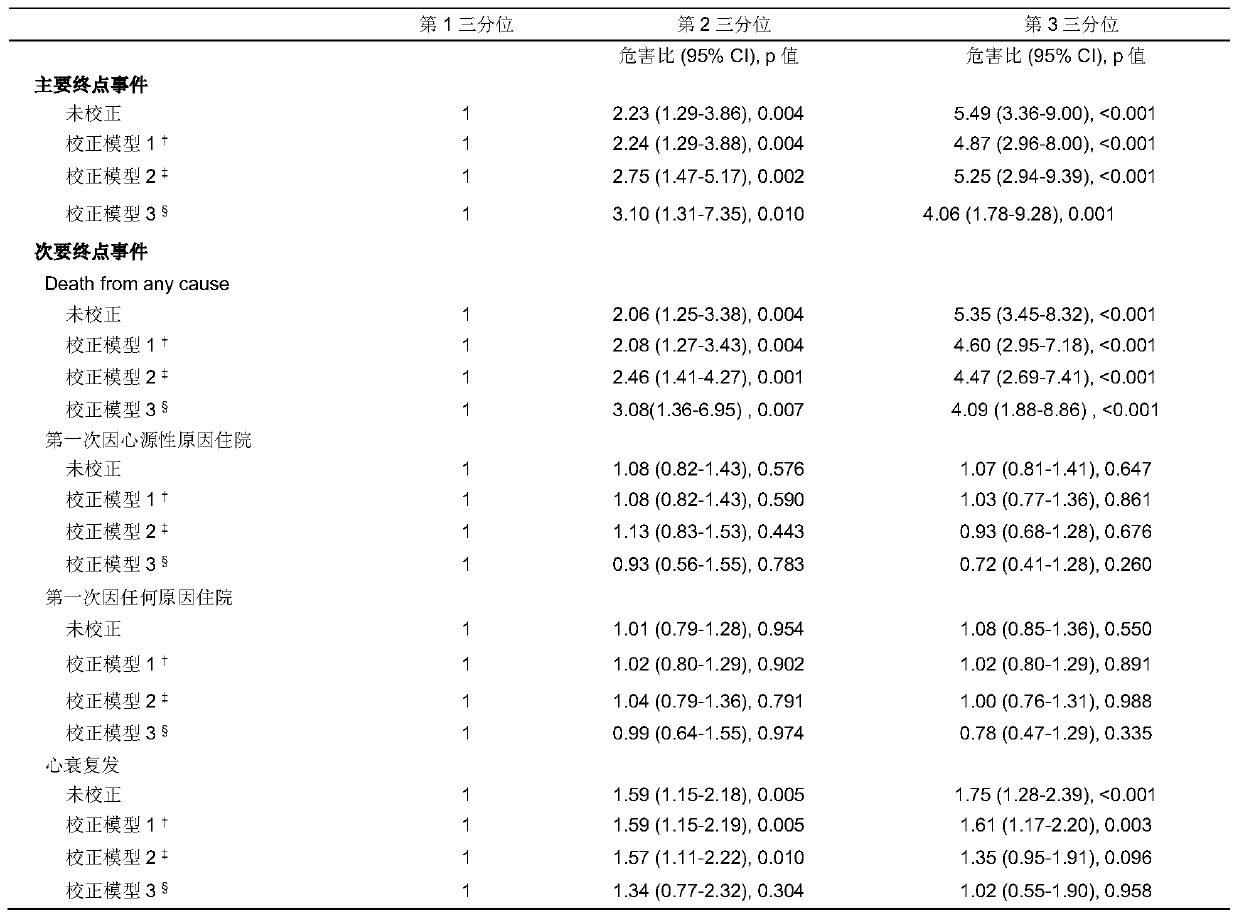
[0042] Increased risk of adverse cardiovascular events in chronic heart failure patients with high plasma Neu5A levels
[0043] Long-term follow-up was carried out on the above-mentioned research population. During the five-year follow-up, only 2 (0.2%) were lost to follow-up. A total of 182 (16.2%) died, of which 149 (13.3%) died of cardiac causes, 3 (0.30%) underwent heart transplantation; 401 (35.8%) were readmitted, of which 297 (26.5%) were of cardiac causes Reasons for hospitalization; 259 people (23.1%) had recurrent heart failure.
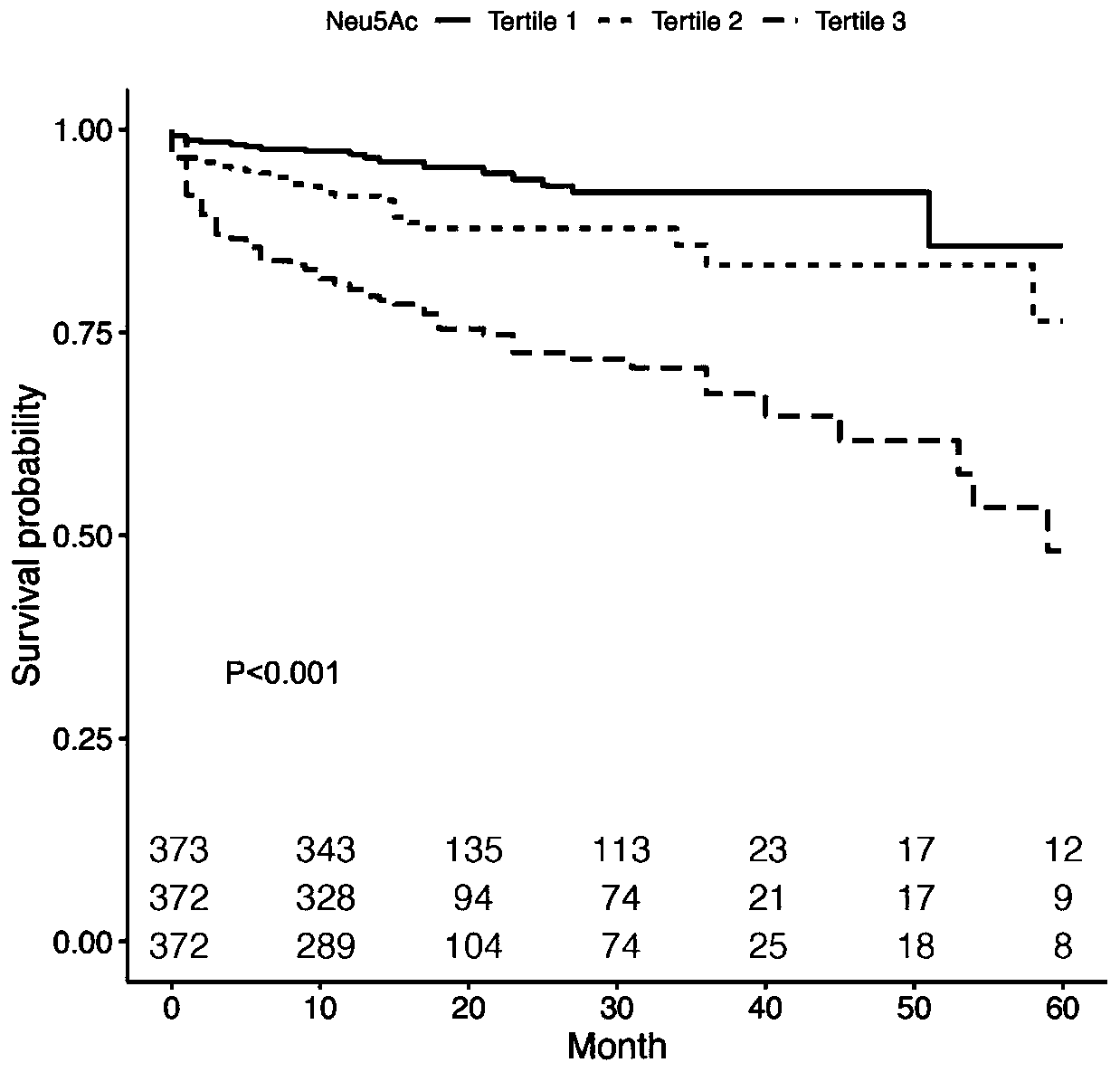
[0044] Statistical analysis methods: Kaplan-Meier survival curves were used to display Neu5Ac tertile levels and clinical endpoint events, and log-rank test was used for statistical evaluation of survival curves. Univariate and multivariate Cox proportional hazards regression with 95% confidence intervals were used to estimate hazard ratios (HRs) according to Neu5Ac tertiles. Results were adjusted for traditional risk factors, including ...
Embodiment 3
[0050] Neu5Ac has stronger predictive value than conventional risk factors in patients with chronic heart failure
[0051] There were 498 people in the study population with hsCRP records, and 2 of them were lost to follow-up. This application studies whether the level of Neu5Ac can help predict the prognosis of patients with chronic heart failure, and whether adding Neu5Ac to the statistical model can improve The predictive value of the model. ROC curve analysis showed that AUC was 0.675, revealing that Neu5Ac was a good predictor of cardiac death and heart transplantation. Logistic regression model was used to construct traditional risk factors and hsCRP models with or without Neu5Ac, and receiver operating characteristic (receiver-operating characteristic, ROC) curve, area under (AUC), net reclassification index (net reclassification indexes, NRI ) and integrated discrimination improvements (IDIs) were used to evaluate the predictive ability of Neu5Ac on the prognosis of p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com