Photoresponse-nitric oxide donor molecule and derivatives and preparing method thereof
A nitric oxide and light-responsive technology, which is applied in the field of medicine, can solve the problems of toxicity, N-nitrosamine derivatives' photosensitivity is not ideal, limitations, etc., and achieve the effect of reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] The present invention also provides a method for preparing a light-responsive nitric oxide donor molecule, comprising:
[0072] Will R 1 CHO, CH 2 (OH)-R 2 -NH 2 React with ethyl methacrylate isocyanate to obtain structure shown in formula (I),
[0073]
[0074] where the R 1 C6-C20 unsubstituted benzyl, C6-C20 substituted benzyl, C5-C20 substituted heteroaryl, C2-C10 alkyl substituted oxyacyl or C5-C10 heteroaryl substituent oxyacyl ;
[0075] The R 2 for-OCH 2 -Ar-(M) a -,
[0076] The Ar is an unsubstituted aryl group of C6-C20, a substituted aryl group of C6-C20 or a heteroaryl group of C4-C15;
[0077] Said M is -OCON(CH 3 )CH 2 -;
[0078] The a is 0 or 1.
[0079] According to the present invention, the present invention will R 1 CHO, CH 2 (OH)-R 2 -NH 2 React with ethyl methacrylate isocyanate to obtain the structure shown in formula (I), wherein, the present invention has no special requirements on the reaction mode, and those skilled in th...
Embodiment 1
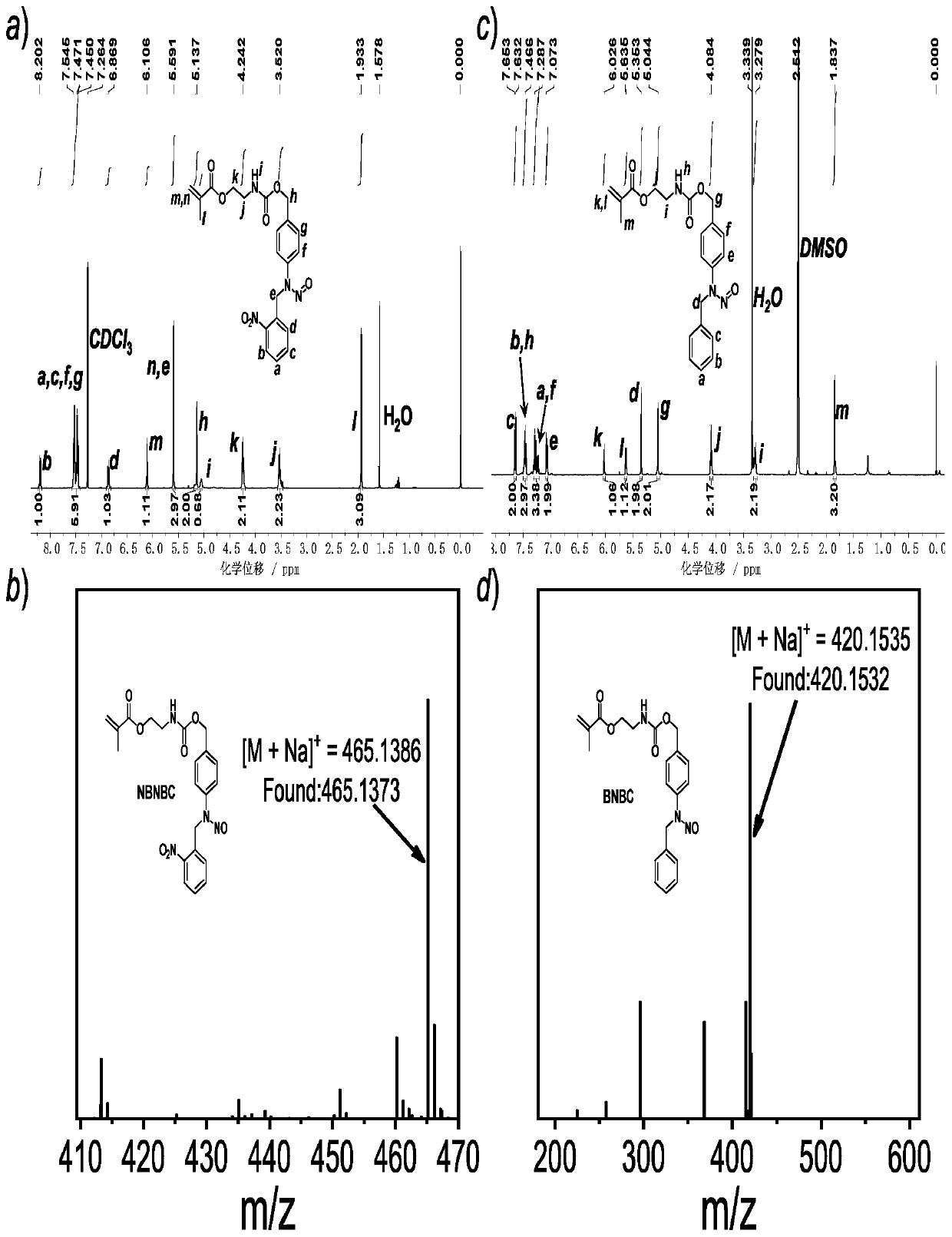
[0105] 1-1) Preparation of photoresponsive nitric oxide donor molecule NBNBC (where R1 is o-nitrobenzaldehyde; R2 is 4-aminobenzyl alcohol)
[0106]
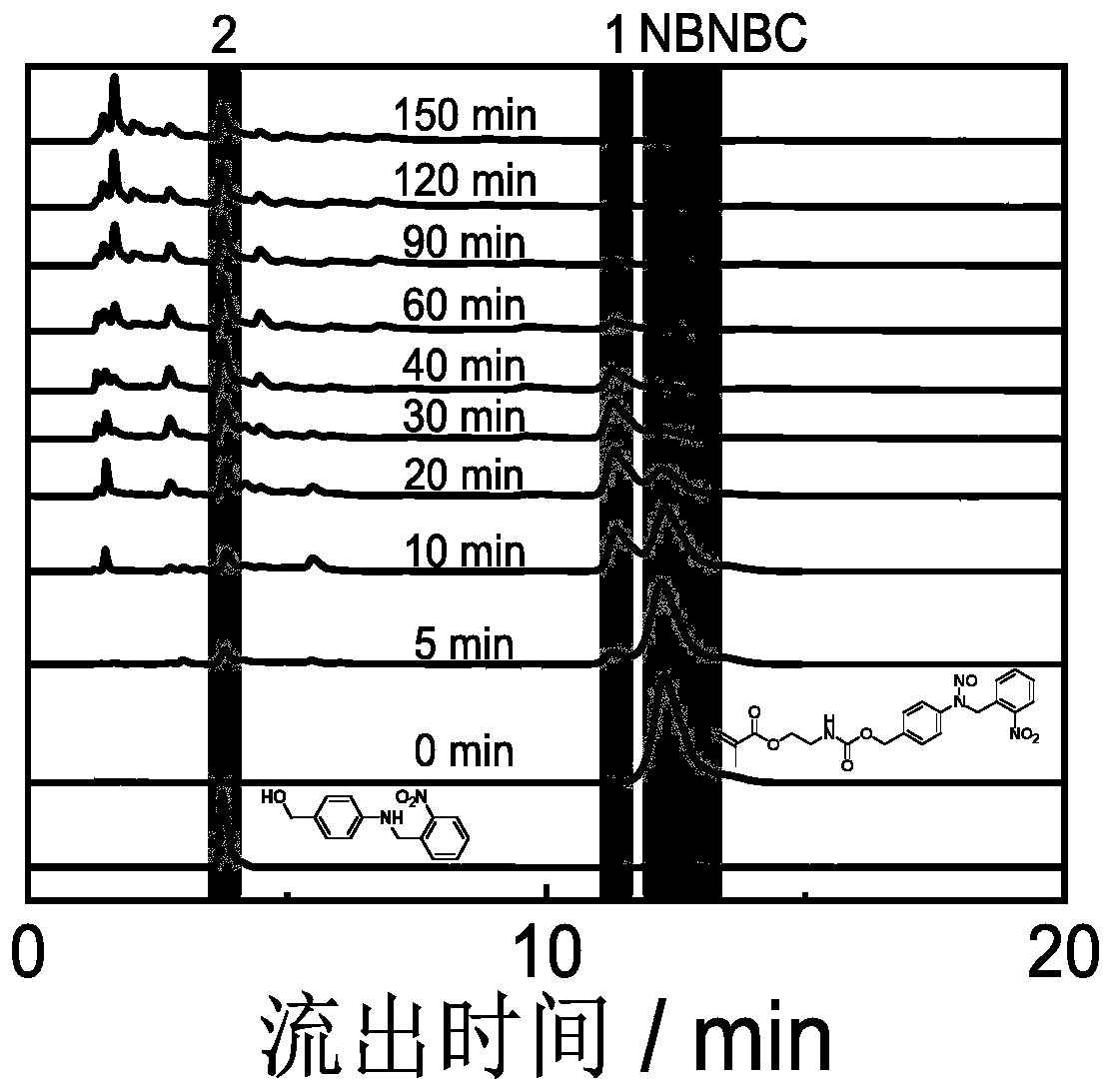
[0107] Preparation method: Dissolve 2g (16.24mmol) of 4-aminobenzyl alcohol (self-made) and 2.45g (16.24mmol) of o-nitrobenzaldehyde in 60mL of ethanol, stir at room temperature for 5min, and precipitate appears. The reaction solution was suction filtered to obtain 2 g (7.80 mmol) of filter cake, which was dissolved in 30 mL of tetrahydrofuran, and 0.295 g (7.80 mmol) of NaBH 4 Stir at room temperature for 2h. Add 5 mL of water to the reaction system, spin dry tetrahydrofuran, extract with dichloromethane, and dry with anhydrous sodium sulfate. Next, 1.03 g (3.97 mmol) of the reduced product was dissolved in 20 mL of glacial acetic acid, and 0.30 g (4.41 mmol) of NaNO2 aqueous solution (60 mL), and the reaction was stirred at room temperature for 20 min. Afterwards, the reaction solution was added to saturated aqueous sodiu...
Embodiment 2
[0122] Embodiment 2 Light releases nitric oxide
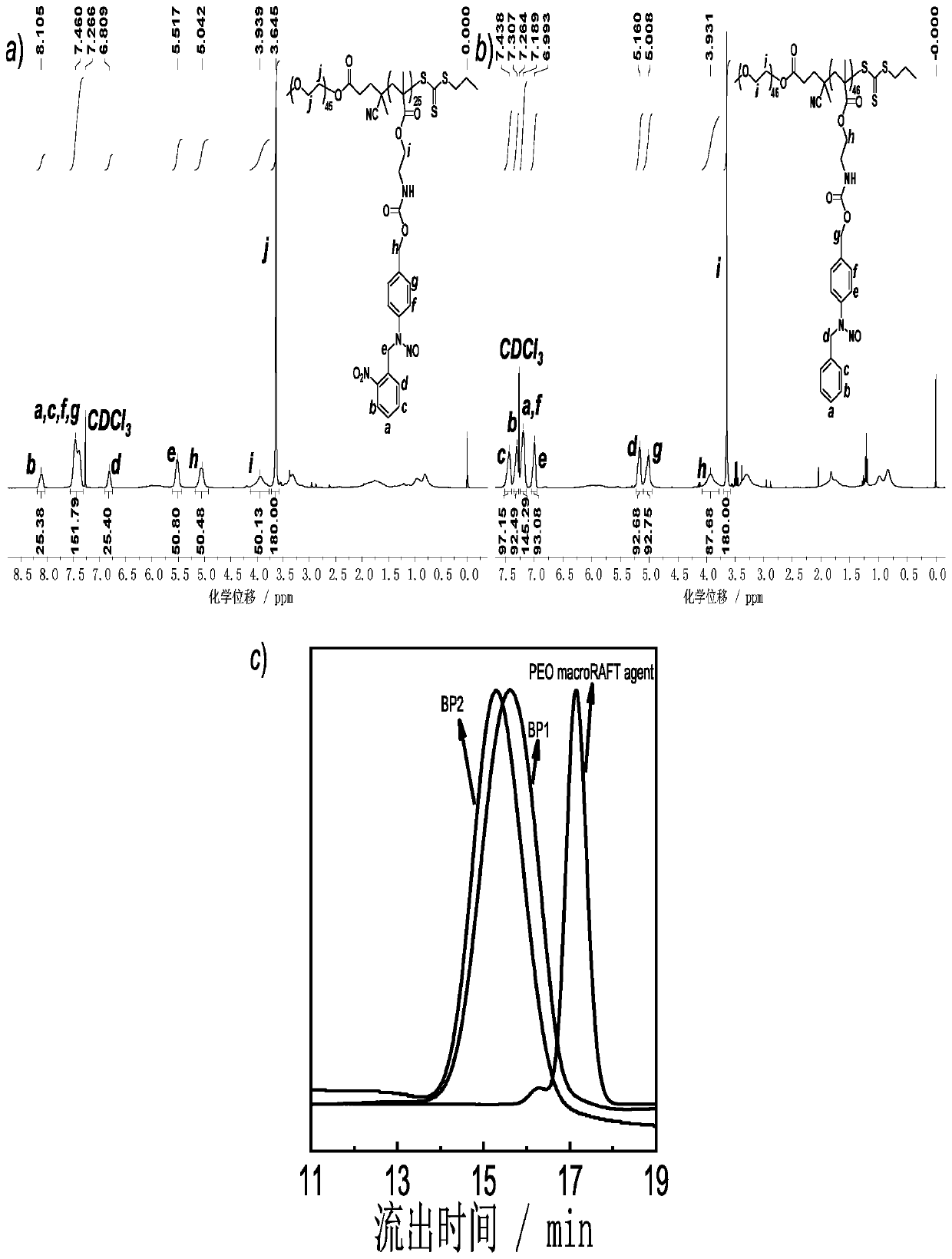
[0123] Nitric oxide release was quantified with Griess reagent. Use a portable UV lamp (365nm, ~4mW / cm 2 ) irradiate the nano-assembly 1 and assembly 2 obtained in Example 1, respectively. Specifically, take the assembly 1 or assembly 2 that has been illuminated for different times and mix it with an equal volume of Griess reagent, place it in the dark for 10 minutes, and test their UV Absorption spectrum, the content of nitric oxide release was quantified by the absorbance at 526nm.
[0124] see results Figure 5 , Figure 5 Curves for quantitative analysis of nitric oxide release under low-dose light conditions for the vesicles obtained from the self-assembly of polymer BP1 and the vesicles obtained from the self-assembly of polymer BP2; , the amount of nitric oxide released tends to be stable, and the assembly releases 80% of nitric oxide. When assembly 2 is illuminated for 7 hours, the assembly releases 26.8% of NO. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com