Quinine compound containing quaternary ammonium groups and preparation method of quinine compound
A technology of quaternary ammonium groups and compounds, which is applied in the field of quinine compounds containing quaternary ammonium groups and their preparation, and can solve problems such as quinine compounds that have not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
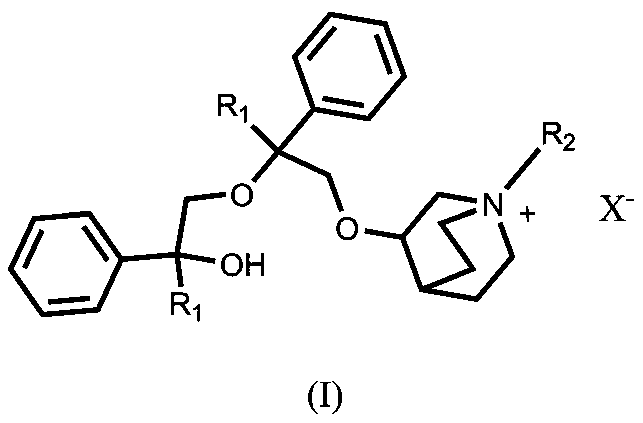
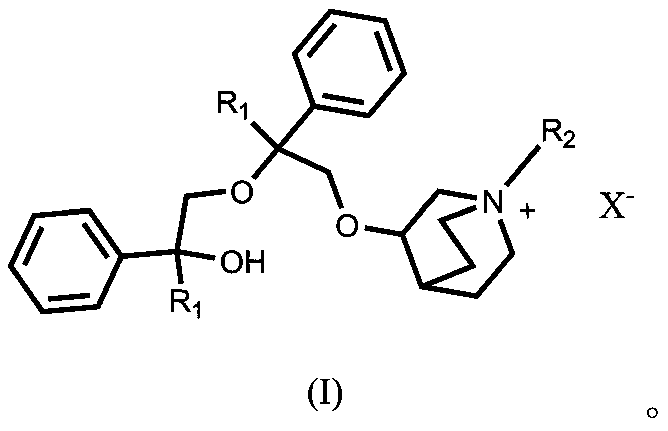
[0023] Preparation of 3-(2-cyclopentyl-2-hydroxy-2-phenylethoxy)quinuclidane
[0024] Add 12.71g (0.1mol) of 3-quinine alcohol into a 500ml there-necked flask, add 100ml of DMSO, stir to dissolve, slowly add 4.2g (0.105mol) of sodium hydride with a content of 60%, raise the temperature to 60°C, stir for 1 hour, and dissolve 20.7 Add g (0.11mol) 1-phenyl-1-cyclopentyl-oxirane in 80mL of DMSO solution, continue stirring at this temperature for 4 hours, cool down to 10°C, add 150ml of water, and extract with methyl tert-butyl ether The organic phases were combined three times, dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure to obtain 26.1 g of light yellow viscous oil.
[0025] Preparation of 3-[2-cyclopentyl-2-phenyl-2-(2-cyclopentyl-2-hydroxy-2-phenyl-ethoxy)ethoxy]quinuclidine
[0026] Add 18.90 g (0.06 mol) of penehyclidine into a 500 ml three-necked flask, add 150 mL of DMSO, add 2.40 g (0.06 mol, 1 eq) of sodium hydride with a conten...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com