Anoxia-responsive nano drug carrier and preparation method and application thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] In this embodiment, the nano-drug is obtained by reacting 2-nitroimidazole with hyaluronic acid to obtain an amphiphilic drug carrier, and then the amphiphilic drug carrier is self-assembled with the hydrophobic drug to obtain a nano-drug.
[0060] Prepare nano-medicine by the following method, specifically comprising the following steps:
[0061] (1) Synthesis of amphiphilic drug carrier
[0062] Weigh 149.6mg of 2-nitroimidazole and dissolve it in 10mL N,N-dimethylformamide, add 280mg of potassium carbonate to the above solution, and add the solution in 1mL N,N-dimethylformamide dropwise under room temperature and magnetic stirring N-Boc-bromoethylamine solution (200mg, 400mg or 600mg) of methyl formamide, transferred to 70°C, 80°C or 90°C oil bath, and reacted for 3h, 4h or 5h. The insoluble matter was removed by suction filtration, washed with methanol, and the clear solution was concentrated by rotary evaporation to obtain an orange-yellow solid. The obtained sol...
Embodiment 2
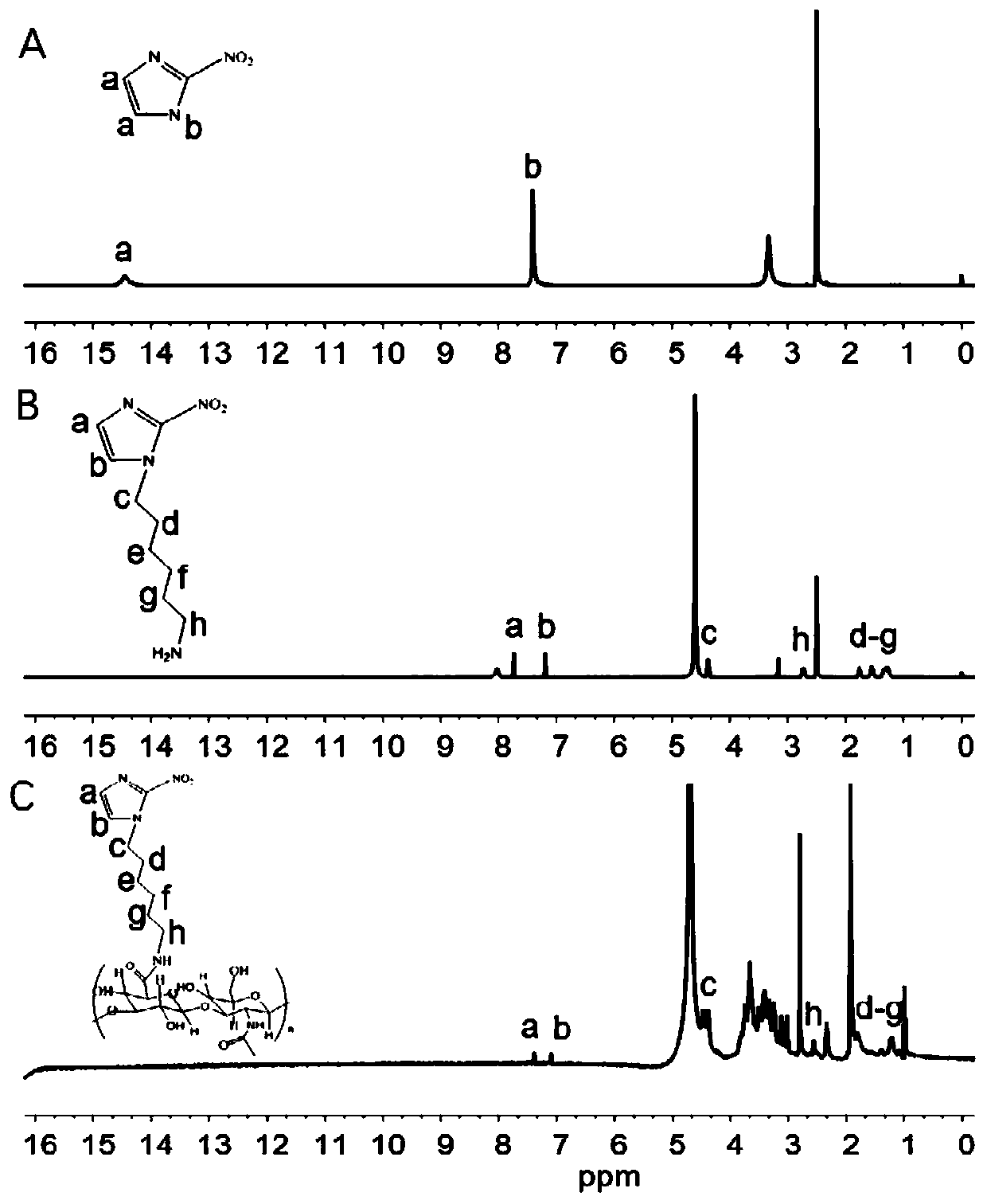
[0067] The intermediate and final products of 2-nitroimidazole grafted on hyaluronic acid were verified by proton nuclear magnetic spectroscopy, and the results are shown in figure 1 .
[0068] figure 1 A is the NMR spectrum of 2-nitroimidazole, after reacting with N-Boc-bromoethylamine, the b position H is replaced to obtain figure 1 The NMR spectrum of B2-nitroimidazole derivatives; the activated -COOH on the hyaluronic acid and the amino group on the 2-nitroimidazole derivatives undergo a condensation reaction to obtain HA-NI, from figure 1 C It can be seen that HA-NI and figure 1 The H of B 2-nitroimidazole derivatives has a similar chemical environment.
Embodiment 3
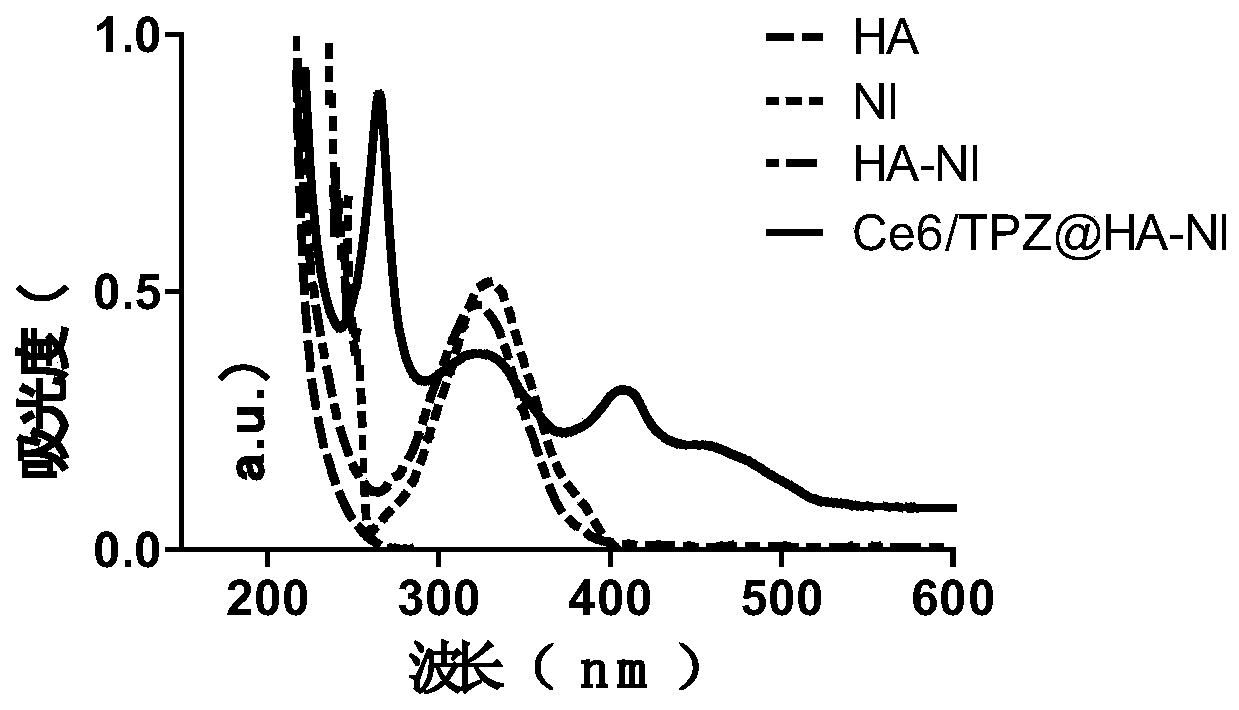
[0070] A UV-Vis spectrophotometer was used to verify the successful grafting of 2-nitroimidazole on hyaluronic acid and the fact that both drugs were encapsulated by the amphiphilic carrier.
[0071] see results figure 2 As shown, in the ultraviolet-visible absorption spectrum, HA-NI shows the ultraviolet absorption peak of NI at 330nm, which further proves the grafting of NI on HA; the ultraviolet-visible absorption spectrum of NPs also shows that HA-NI is at 330nm, Ce6 The peak shape at 400nm and TPZ at 470nm proved that Ce6 and TPZ were simultaneously encapsulated in the HA-NI nanomedicine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com