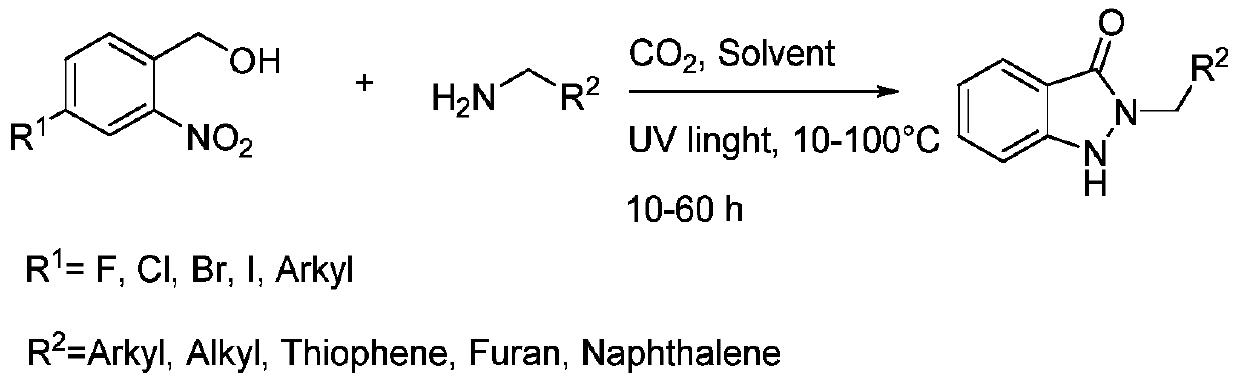
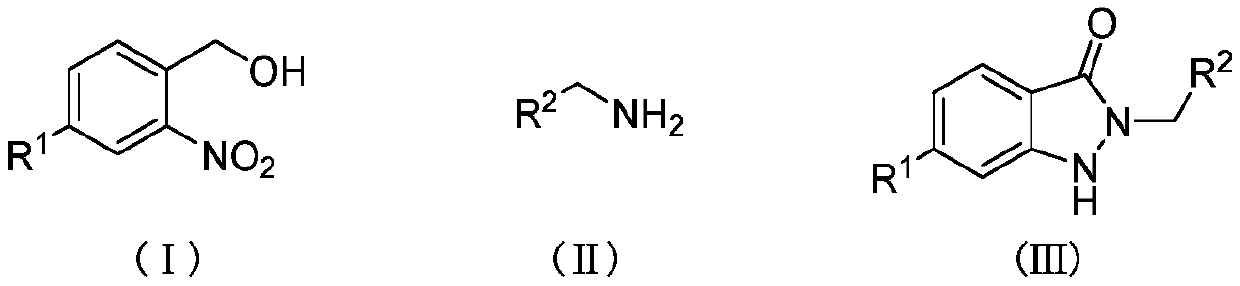
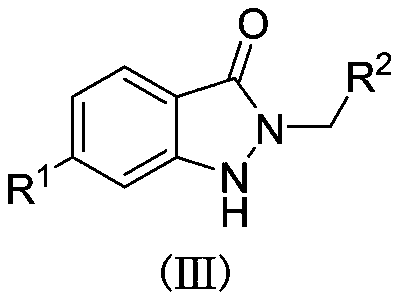
Preparation method for synthesizing indazolinone compound by carbon dioxide promotion and photocatalyst-free photo-induction
A technology of indazolinone and carbon dioxide, applied in the field of catalytic organic synthesis, can solve the problems of easy oxidation, many operation steps, and unfriendly environment of transition metals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0018] Add 0.2mmol I (where R 1 =H), 0.24mmol II (wherein R 2 =C 6 h 5 ) and 4mLTHF, the gas atmosphere is a carbon dioxide atmosphere, under the ultraviolet light at 25 ℃ for 24h, after the reaction is completed, filter, concentrate, and get III through chromatographic separation (wherein R 1 = H; R 2 =C 6 h 5 ), the yield was 91%.
preparation example 2
[0020] Add 0.2mmol I (where R 1 =H), 0.24mmol II (wherein R 2 =3-F-C 6 h 5 ) and 4mL THF, the gas atmosphere is a carbon dioxide atmosphere, under the ultraviolet light at 25 ℃ for 24h, after the reaction is completed, filter, concentrate, and obtain III through chromatographic separation (wherein R 1 = H; R 2 =3-F-C 6 h 5 ), with a yield of 75%.
preparation example 3
[0022] Add 0.2mmol I (where R 1 =H), 0.24mmol II (wherein R 2 = 4-Cl-C 6 h 5 ) and 4mL THF, the gas atmosphere is a carbon dioxide atmosphere, under the ultraviolet light at 25 ℃ for 24h, after the reaction is completed, filter, concentrate, and obtain III through chromatographic separation (wherein R 1 = H; R 2 = 4-Cl-C 6 h 5 ), the yield was 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com