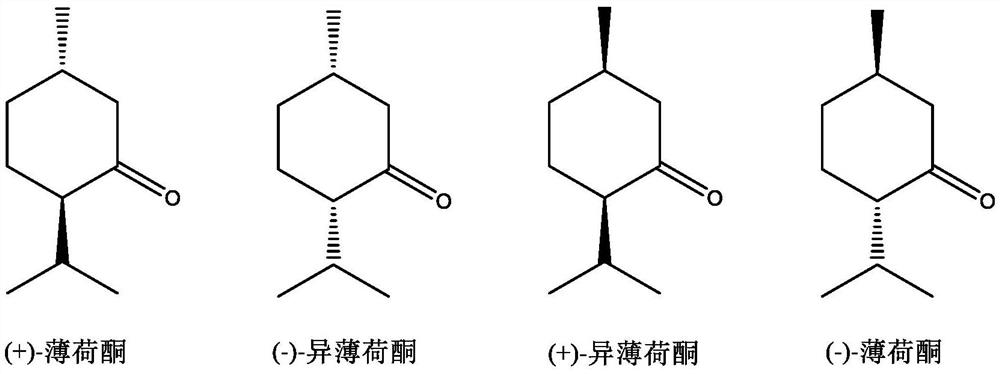
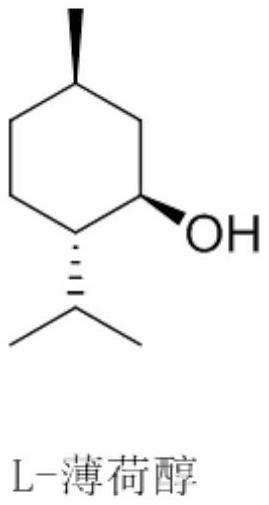
A kind of method for preparing optically pure 1-menthone and catalyst for the method
A technology of menthone and oxidation catalyst, which is applied in the direction of physical/chemical process catalysts, chemical instruments and methods, and oxidation to prepare carbonyl compounds, etc., which can solve the problems of limited turnover increase, short catalyst life, and adverse environmental effects, etc., to increase life , high stability, high activity and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Under argon atmosphere, 26.1 mg of salen manganese compound (1) (4-tBu-Salen Mn, purchased from Aldrich Company), 15.42 g of racemic menthol (ee=0%, L-menthol / D-menthol= 1:1 (mol)) was dissolved in 15 mL of acetonitrile, transferred to a 50 mL reaction kettle, turned on stirring, cooled to 0 °C, slowly added dropwise 5.7 g of 30% hydrogen peroxide solution, reacted at 0 °C for 1 h, and then used gas chromatography The conversion rate of L-menthol was measured to be 99.9%, the product was L-menthone, the optical purity was 99ee%, and the yield of L-menthol was 99.8% based on the L-menthol in the raw material.
Embodiment 2
[0060] Under an argon atmosphere, 32 mg of salen manganese compound (1) and 15.42 g of L-menthol (ee=98%) were dissolved in 15 mL of acetonitrile, transferred to a 50 mL reaction kettle, stirred, cooled to 0 °C, and slowly added dropwise. 17 g of a 30% hydrogen peroxide solution was reacted at 0 °C for 3 hours, and the conversion rate of L-menthol was measured by gas chromatography to be 99.9%. The product was L-menthone, and the optical purity was 99ee%. The yield of L-menthone was 99.8%.
Embodiment 3-5
[0062] Under argon atmosphere, the salen manganese compounds of structural formula (2)-(4) (4-tBu-2-Me-SalenMn(2), 2,4-di-tBu-SalenMn(3), 4- Naphthalen-Salen Mn (4) was purchased from Aldrich Company) 0.05mmol, 15.22g racemic menthol (ee=0%, L-menthol / D-menthol=1:1 (mol)) was dissolved in 15mL acetonitrile, And transferred to a 50mL reaction kettle, turned on stirring, cooled to 0°C, slowly added dropwise 5.7g of 30% hydrogen peroxide solution, reacted at 0°C for 5h, and used gas chromatography to measure the conversion rate of L-menthol in the raw materials and the ratio of the raw materials to the raw materials. The yield of L-menthol in the L-menthol and the optical purity of the product L-menthone are shown in Table 1.
[0063] Table 1 Reaction conditions and results
[0064]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com