Preparation method of alvimopan
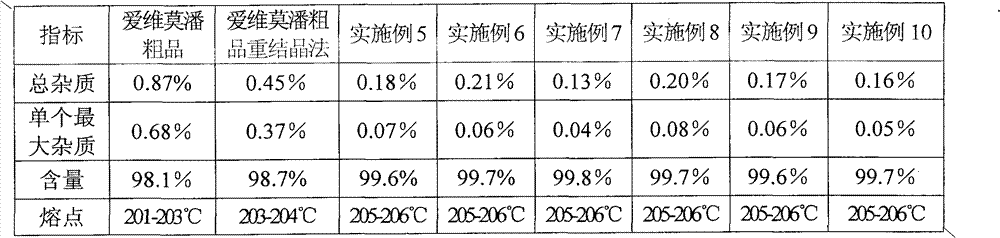
A technology for crude products of Avimopan and Vimopan, which is applied in the field of pharmaceutical chemical preparation, can solve the problems of harsh alkaline hydrolysis reaction conditions, unsuitable for industrial production, and difficult separation and purification, and achieve high reaction yield and stable configuration , the effect of cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of Alvimopan Crude
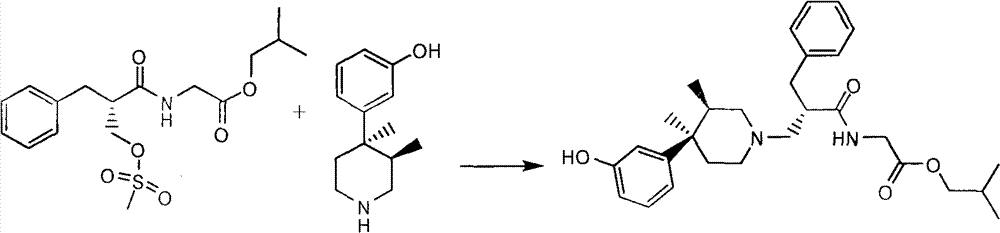
[0031] (3R,4R)-3-(3,4-Dimethyl-4-piperidinyl)phenol 60g (0.292mol), (S)-2-methylsulfonylmethylphenylpropionyl-glycine isobutyl ester Add 144g (0.388mol), 180ml of triethylamine, and 3000ml of toluene into a three-necked flask, heat and reflux for 6 hours, after the reaction is complete, cool to room temperature, add 1200ml of water and stir, separate the organic layer, dry over anhydrous sodium sulfate, filter, and reduce Concentrate under pressure to exhaust the solvent to obtain 88 g of Avibutyrate, properties: pale yellow oil, yield: 63.8%. After nuclear magnetic resonance, the data are as follows:
[0032] 1 HNMR (300MHZ, DMSO): 0.68~0.71(d, 3H), 0.86~0.89(m, 6H), 1.02~1.05(d, 2H), 1.31(d, 3H), 1.60~1.82(s, 1H), 1.82~1.95(s, 1H), 2.20~2.40(m, 2H), 2.67~2.98(m, 3H), 3.00~3.30(dd, 4H), 3.75~3.95(dd, 4H), 6.61~6.63(m , 3H), 7.11 (m, 1H), 7.23-7.30 (m, 5H), 9.00-9.05 (s, 1H), 9.33 (s, 1H).
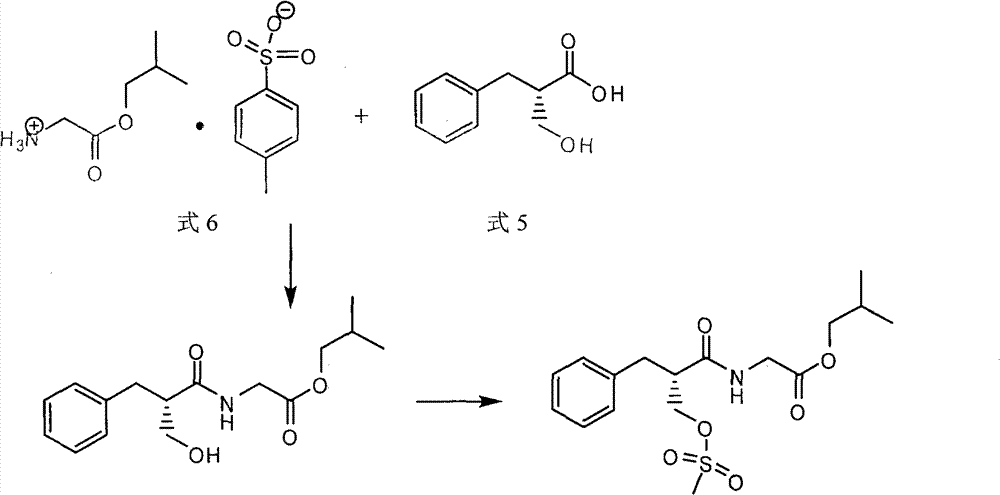
[0033]Take 88g of Evitamin isobutyl ester...
Embodiment 2
[0035] Preparation of Alvimopan Crude
[0036] (3R,4R)-3-(3,4-Dimethyl-4-piperidinyl)phenol 30g (0.146mol), (S)-2-methylsulfonylmethylphenylpropionyl-glycine isobutyl ester Add 55g (0.148mol), 150ml of diethylamine, and 1500ml of toluene into a three-necked flask, heat and reflux for 6 hours, after the reaction is complete, cool to room temperature, add 600ml of water and stir, separate the organic layer, dry over anhydrous sodium sulfate, filter, reduce Concentrate under pressure to exhaust the solvent to obtain 34 g of Avibutyrate, properties: pale yellow oil, yield: 48.4%. Take 34g of Evitamin Isobutyl Ester and add it to a 2000ml three-necked flask, add 850ml of ethanol and 100ml of water, add 210ml of 1N sodium hydroxide solution dropwise under stirring at room temperature, drop it in 20 minutes, stir at room temperature for 3 hours, concentrate under reduced pressure to remove ethanol, and the residual Add 200ml of absolute ethanol to the mixture, add 6N hydrochloric ac...
Embodiment 3
[0038] Preparation of Alvimopan Crude
[0039] (3R,4R)-3-(3,4-Dimethyl-4-piperidinyl)phenol 30g (0.146mol), (S)-2-methylsulfonylmethylphenylpropionyl-glycine isobutyl ester Add 81g (0.218mol), 300ml of triethylamine, and 1200ml of toluene into a three-neck flask, heat and reflux for 6 hours, after the reaction is complete, cool to room temperature, add 600ml of water and stir, separate the organic layer, dry over anhydrous sodium sulfate, filter, reduce Concentrate under pressure to exhaust the solvent to obtain 42 g of Avibutyrate, properties: pale yellow oil, yield: 59.8%. Take 42g of Evitamin Isobutyl Ester and add it to a 2000ml three-neck flask, add 850ml of ethanol and 100ml of water, add 90ml of 1N sodium hydroxide solution dropwise under stirring at room temperature, drop it in 20 minutes, stir at room temperature for 3 hours, concentrate under reduced pressure to remove ethanol, and the residual Add 200ml of absolute ethanol to the mixture, add 6N hydrochloric acid d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com