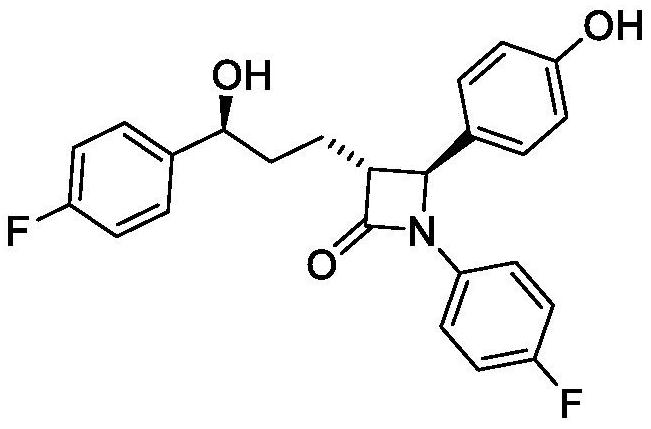
Synthesis process of ezetimibe bulk drug
A synthesis process, ezetimibe technology, applied in the field of synthesis of small molecule cardiovascular drugs, can solve the problems of low purity of hand-reduced products and unsuitability for industrial production, and achieve high product content and optical purity, easy operation and reaction conditions Gentle and manageable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
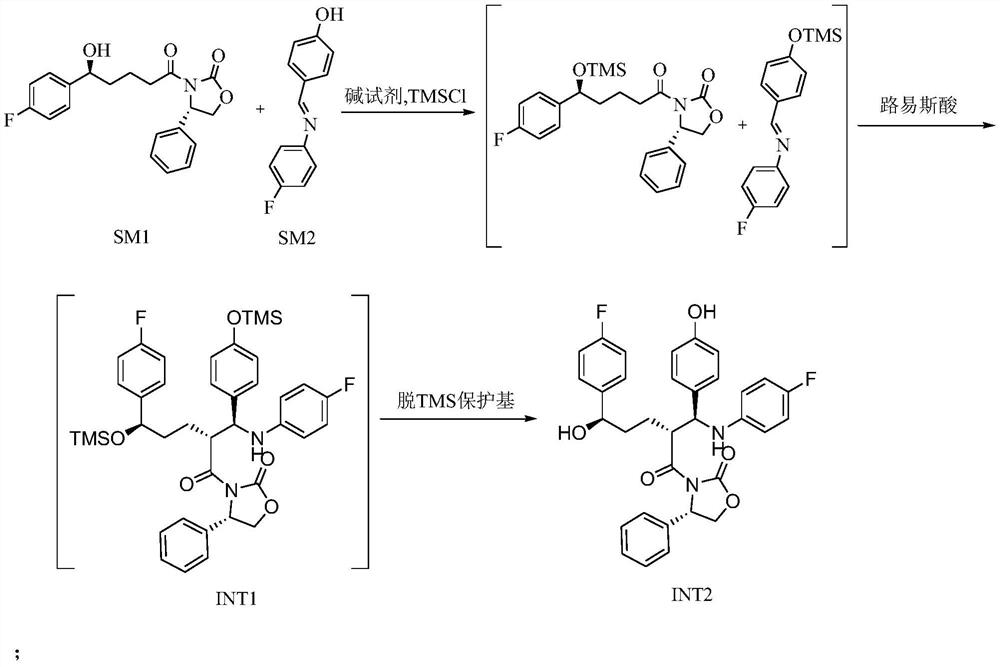
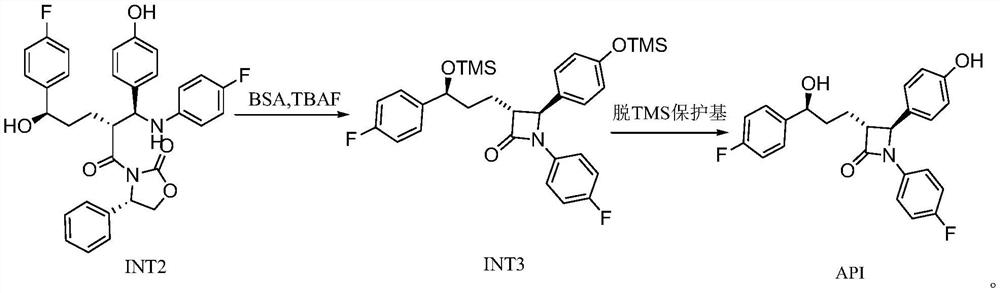
[0035] 1. Preparation of Intermediate 2 (INT2)
[0036] In the reaction kettle, dichloromethane (1 L), SM1 (200 g), SM2 (180 g) were added successively, and the reaction was stirred under nitrogen protection. Cool down to -10~0°C, add DIPEA (290g), continue to cool down to -20~-10°C, slowly add TMSCl (183g) dropwise, control the temperature of the dripping liquid not to exceed 0°C, the solids in the system gradually dissolve until completely dissolved , as a pale yellow solution. After dropping, control the liquid temperature to -10-0°C for reaction, and detect with TLC (developing solvent: PE / EA=2 / 1) until the SM1 reaction is complete.
[0037] Cool the reaction system to -20~-10°C, add titanium tetrachloride (128g) and 600ml dichloromethane dropwise under stirring, control the liquid temperature not to exceed -20°C, after the addition, slowly add tetraisopropyl titanate dropwise For ester (64g), control the liquid temperature not to exceed -20°C. After the drop is complete...
Embodiment 2
[0049] 1. Preparation of Intermediate 2 (INT2)
[0050] In the reaction kettle, dichloromethane (5L), SM1 (1.00kg), SM2 (903g) were added sequentially, and the reaction was stirred under nitrogen protection. Cool down to -10-0°C, add DIPEA (1.45kg), continue to cool down to -20--10°C, slowly add TMSCl (912g) dropwise, control the temperature of the dripping solution not to exceed 0°C, and gradually dissolve the solids in the system until completely dissolved as a light yellow solution. After dropping, control the liquid temperature to -10-0°C for reaction, and detect with TLC (developing solvent: PE / EA=2 / 1) until the SM1 reaction is complete.
[0051] Cool the reaction system to -20~-10°C, add titanium tetrachloride (637g) and 3L dichloromethane mixture dropwise under stirring, control the liquid temperature not to exceed -20°C, after the addition is complete, slowly add tetraisopropyl titanate dropwise For ester (318g), control the liquid temperature not to exceed -20°C. Af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com