Catalyst system for preparing hydrocarbons and use thereof
A catalyst, a technology for producing hydrocarbons, applied in molecular sieve catalysts, chemical instruments and methods, catalyst activation/preparation, etc., can solve the problems of inconvenient filling, low selectivity of target products aromatics, low CO conversion rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
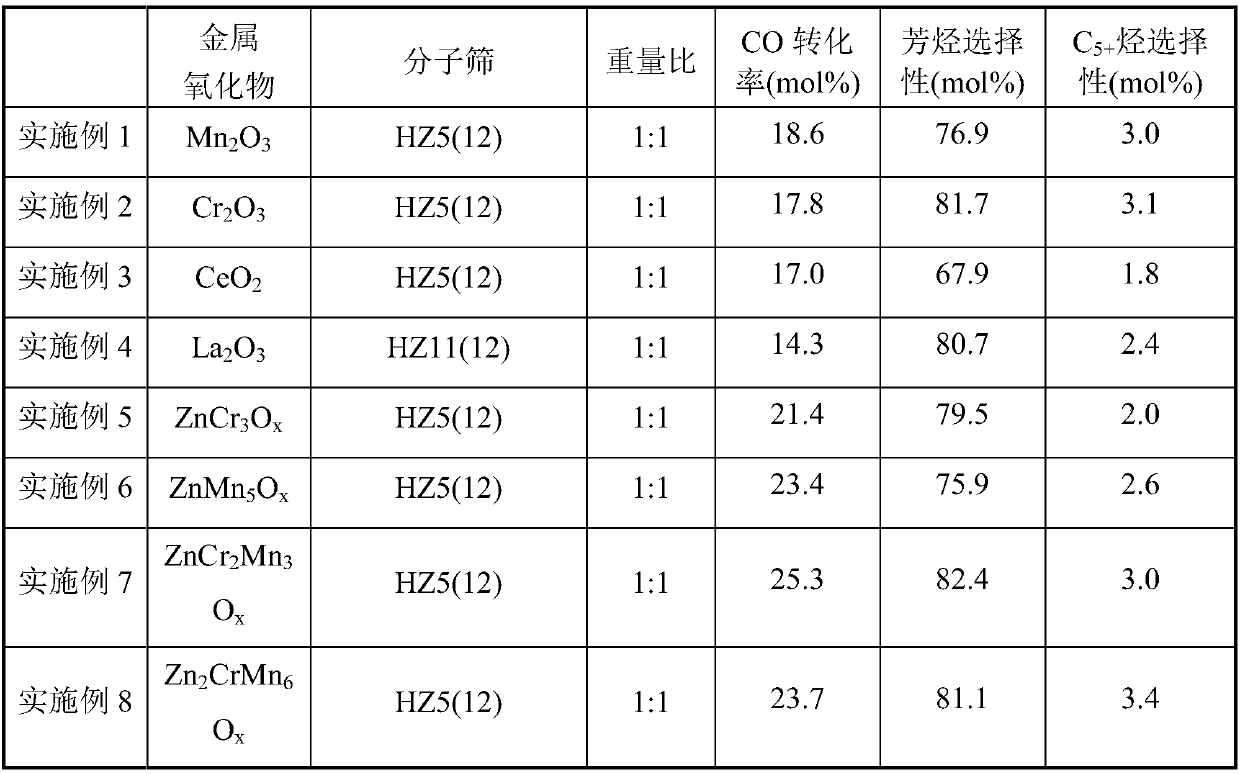
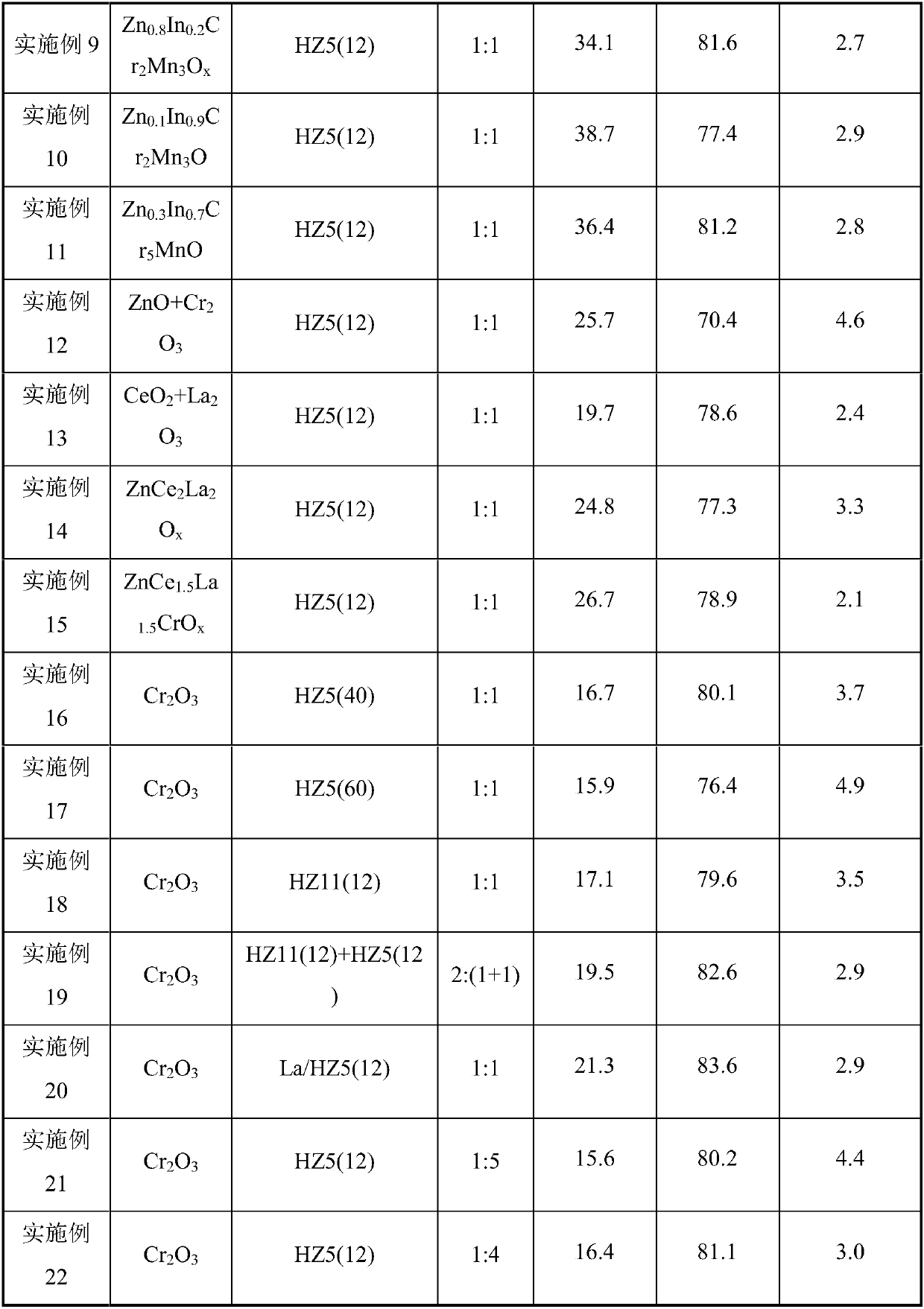
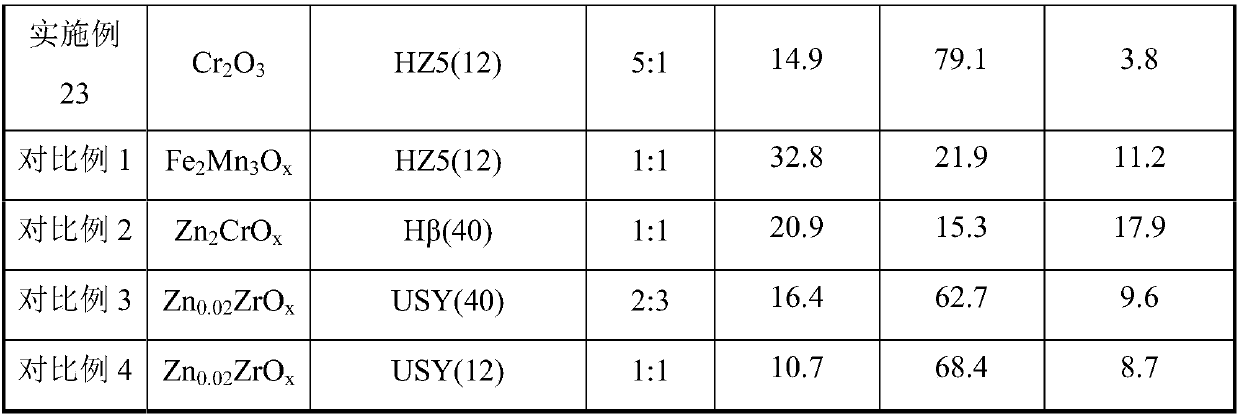
[0052] Preparation of Manganese Oxide Mn by Precipitation Method 2 o 3 , the HZSM-5 molecular sieve with a Si / Al ratio of 12 was synthesized by a hydrothermal method, and the obtained molecular sieve was denoted as HZ5(12); the Mn 2 o 3 , HZ5(12) powder is mechanically mixed according to the mass ratio of 1:1, then granulated and crushed to obtain catalyst particles of 20-40 mesh. Weigh 1.5g of catalyst particles and load them into the reactor. At a reaction temperature of 395°C and a pressure of 2.0MPa, the feed gas H 2 / CO ratio 1.0, airspeed 2000h -1 Catalyst evaluation was carried out under the conditions. Before the reaction, the catalyst was heated with H at 395°C 2 Pretreatment 2h.
[0053] Feed gas H 2 / CO / N 2 , the product was analyzed online by gas chromatography, where N 2 Quantitative analysis of the product was achieved for the internal standard. The product is separated by three columns, one of which is a hayesep-Q packed column, and the separated prod...
Embodiment 2
[0055] Preparation of Chromium Oxide Cr by Precipitation Method 2 o 3 , the HZSM-5 molecular sieve with a Si / Al ratio of 12 was synthesized by a hydrothermal method, and the obtained molecular sieve was denoted as HZ5(12); the Cr 2 o 3 , HZ5(12) powder is mechanically mixed according to the mass ratio of 1:1, then granulated and crushed to obtain catalyst particles of 20-40 mesh. Weigh 1.5g of catalyst particles and load them into the reactor. At a reaction temperature of 395°C and a pressure of 2.0MPa, the feed gas H 2 / CO ratio 1.0, airspeed 2000h -1 Catalyst evaluation was carried out under the conditions. Before the reaction, the catalyst was heated with H at 395°C 2 Pretreatment 2h. The results of CO conversion and product selectivity are shown in Table 1.
Embodiment 3
[0057] Preparation of Cerium Oxide CeO by Hydrothermal Method 2 , the HZSM-5 molecular sieve with a Si / Al ratio of 12 was synthesized by a hydrothermal method, and the obtained molecular sieve was denoted as HZ5(12); the CeO 2 , HZ5(12) powder is mechanically mixed according to the mass ratio of 1:1, then granulated and crushed to obtain catalyst particles of 20-40 mesh. Weigh 1.5g of catalyst particles and load them into the reactor. At a reaction temperature of 395°C and a pressure of 2.0MPa, the feed gas H 2 / CO ratio 1.0, airspeed 2000h -1 Catalyst evaluation was carried out under the conditions. Before the reaction, the catalyst was heated with H at 395°C 2 Pretreatment 2h. The results of CO conversion and product selectivity are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com