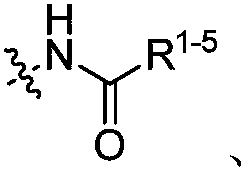
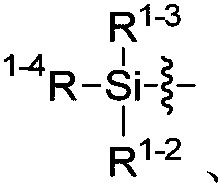Fluorine methyl -containing compound and preparation method thereof
A compound and methyl technology, which is applied in the field of fluoromethyl-containing compounds and their preparation, can solve the problems of expensive preparation methods, poor functional group compatibility, and difficult preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0172] The preparation method of nickel complex containing ligand coordination:
[0173] (4,4’-di-tBu-bpy)NiCl 2 :(Angew.Chem.Int.Ed.2016,55,5837-41.)
[0174]
[0175] Add anhydrous NiCl to a 100mL three-neck flask 2 (129.6 mg, 1.0 mmol) in 13 mL of an ethanolic solution, to which was added 7 mL of an ethanolic solution of 4,4'-ditBu-bpy (271 mg, 1.0 mmol), the reaction solution was heated to reflux for 10 h, the reaction solution was filtered, and the filtrate was concentrated to obtain Green solid 358 mg (90% yield), the solid was recrystallized from methanol.
[0176] (4,4’-di-NH 2 -bpy)NiCl 2 :
[0177]
[0178] Add anhydrous NiCl to the 25mL reaction flask 2 (129.6 mg, 1.0 mmol) in 5 mL of methanol, to which was added 4,4'-ditNH 2 - 5 mL of methanol solution of bpy (186.2 mg, 1.0 mmol), the reaction solution was heated at 80°C for 10 h, the reaction solution was filtered, and the filtrate was concentrated to obtain a green solid. The solid was recrystallize...
Embodiment 1
[0186]
[0187] Method 1: 36.7 mg of the target product was obtained with a yield of 90%; the purity was identified by hydrogen spectrum as more than 95%.
[0188] 1 H NMR (400MHz, CDCl 3 )δ7.72-7.68(m, 2H), 7.64-7.59(m, 4H), 7.52-7.47(m, 2H), 7.44-7.49(m, 1H), 6.71(t, J=56.4Hz, 1H) . 19 F NMR (376MHz, CDCl 3 )δ-110.3(d, J=56.5Hz, 2F). 13 C NMR (125.7MHz, CDCl 3 )δ143.7(t,J=2.0Hz),140.2,133.2(t,J=22.4Hz),128.9,127.9,127.4,127.2,126.0(t,J=6.0Hz),114.7(t,J=238.5 Hz).
Embodiment 2
[0190]
[0191] Method 1: 36 mg of the target product was obtained with a yield of 87%; the purity was identified by hydrogen spectrum as more than 95%.
[0192] 1 H NMR (400MHz, CDCl 3 )δ7.76(s,1H),7.73(d,J=7.3Hz,1H),7.63(d,J=7.5Hz,2H),7.59–7.45(m,4H),7.41(t,J=7.3 Hz,1H),6.73(t,J=56.5Hz,1H). 19 F NMR (376MHz, CDCl 3 )δ-110.6(d, J=56.5Hz, 2F). 13 C NMR (101MHz, CDCl 3 )δ141.8,140.1,134.9(t,J=22.2Hz),129.4(t,J=1.9Hz),129.2,128.9,127.8,127.2,124.3(t,J=6.0Hz),124.3(t,J=6.0 Hz), 114.7(t, J=238.9Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com