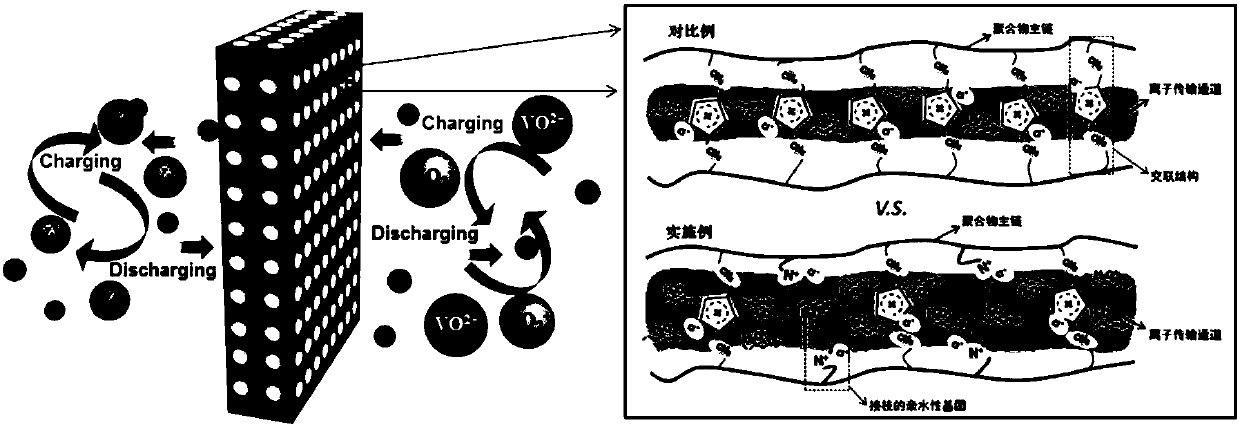
Ionic conduction membrane for flow battery and preparation and application thereof
A technology for ion-conducting membranes and flow batteries, applied to ion-conducting membranes and their applications in flow batteries, can solve problems such as reduced membrane stability, and achieve the effects of improving selectivity, improving conduction, and improving preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 10 g of chloromethylated polysulfone were dissolved in 400 g of DMAc (wherein the 1 The degree of chloromethylation of the prepared chloromethyl polysulfone as measured by HNMR is 1.3), stirred for 12 hours to form a uniform polymer solution, left to defoam, scraped on a dust-free glass plate, placed at 50 ° C, 10 minutes in a constant temperature and humidity chamber with a relative humidity of 100%, soak in deionized water for 12 hours after removing the solvent on the surface. Soak the porous ion-conducting membrane prepared above in 40°C, 5% trimethylamine aqueous solution, take it out after 5 minutes, wash it with deionized water, then soak the above-mentioned porous ion-conducting membrane grafted with trimethylamine in 40°C, 10% trimethylamine imidazole aqueous solution, take it out after 72h, wash with deionized water, soak in 3mol / L H 2 SO 4 12h in aqueous solution.
[0035] Use the porous ion-conducting membrane prepared by the above method to assemble an a...
Embodiment 2
[0037] The material characterization method and basement membrane preparation and characterization method were the same as in Example 1. The porous ion-conducting membrane prepared above was soaked in 5% pyridine aqueous solution at 40°C, taken out after 5 minutes, washed with deionized water, and then the above-mentioned grafted pyridine Soak the porous ion-conducting membrane in 10% imidazole aqueous solution at 40°C, take it out after 72 hours, wash it with deionized water, soak it in 3mol / L H 2 SO 4 12h in aqueous solution.
[0038] The battery assembly and test conditions are the same as in Example 1. In the charge and discharge experiment, the current density is 80mA / cm 2 , image 3 It can be seen that its coulombic efficiency is 97.9%, its voltage efficiency is 91%, and its energy efficiency is 89.1%. 2 Under the current density, the energy efficiency is maintained at 81% after 1000 cycles of charging and discharging.
Embodiment 3
[0040] 4 grams of chloromethylated polysulfone (wherein 1 HNMR records that the degree of chloromethylation of the prepared chloromethyl polysulfone is 1.2) Dissolved in 16 grams of DMAc, stirred for 12h, formed a uniform polymer solution, added 0.5g piperidine, stirred into a uniform solution, and statically Put it in defoaming, scrape it on a dust-free glass plate, put it on a hot stage at 50°C to evaporate the solvent to form a dense film (film thickness is about 32μm), and then soak the ion-conducting membrane prepared above to 40°C, 10% imidazole aqueous solution, take it out after 72h, wash with deionized water, soak in 3mol / L H 2 SO 4 12h in aqueous solution.
[0041] The battery assembly and test conditions are the same as in Example 1. In the charge and discharge experiment, the current density is 80mA / cm 2 , image 3 It can be seen that its coulombic efficiency is 98.6%, the voltage efficiency is 91.2%, and the energy efficiency is 89.9%. 2 Under the current den...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com