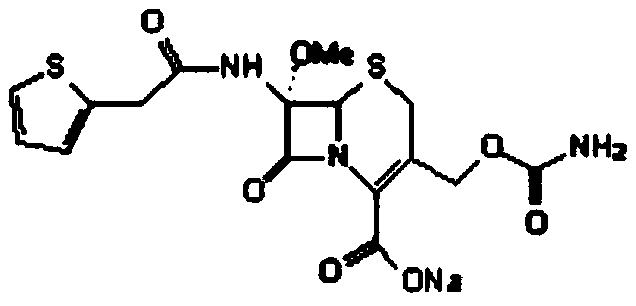
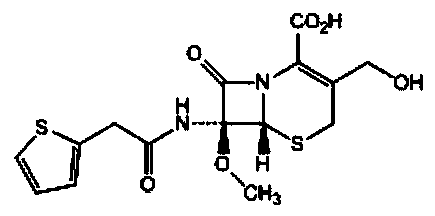
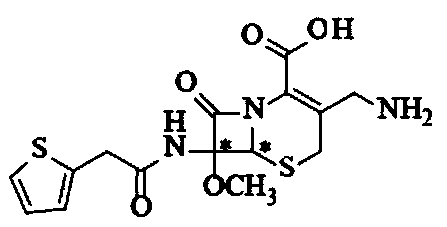
Synthesis method of cefoxitin sodium
A technology of cefoxitin sodium and a synthetic method, which is applied in the field of synthesis of cefoxitin sodium, can solve the problems of high impurity content, reduced quality, and reduced yield of cefoxitin, so as to achieve stable product quality, reduce side reactions and Hydrolysis, the effect of reducing product impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] The cefoxitin involved in the present embodiment 1 is synthesized through the following steps:
[0066] Synthesis of S1.7-a-methoxycefalotin cyclohexylamine salt:
[0067] In the flask at room temperature, add 260mL of dichloromethane, 20ml of methanol and 20ml of tetrahydrofuran, add 25g of cephalothinic acid under stirring, cool to -95°C with liquid nitrogen, add the prepared sodium methoxide solution (80ml of methanol and 40ml of dichloromethane , sodium methoxide 15g). Control the temperature at -86~-89°C, drop the temperature to -95°C after adding, add 8g of tert-butyl hypochlorous acid dropwise, drop the temperature to -90°C after the addition, and react for 45 minutes.
[0068] After the reaction was completed, 6 g of sodium metabisulfite was added and stirred for 10 minutes. Add 18ml of 80% acetic acid and heat up to -65°C. Add 300 ml of 10% sodium chloride aqueous solution, stir evenly after adding, and measure the cephalothin residue ≤ 5% by HPLC. Heat the...
Embodiment 2
[0079] The cefoxitin involved in the present embodiment 2 is synthesized through the following steps:
[0080] Synthesis of S1.7-a-methoxycefalotin cyclohexylamine salt:
[0081] In the flask at room temperature, add 260mL of dichloromethane, 20ml of methanol and 20ml of tetrahydrofuran, add 25g of cephalothinic acid under stirring, cool to -95°C with liquid nitrogen, add the prepared sodium methoxide solution (80ml of methanol and 40ml of dichloromethane , sodium methoxide 15g). Control the temperature at -86~-89°C, drop the temperature to -95°C after adding, add 15g of tert-butyl hypochlorous acid dropwise, drop the temperature to -90°C after the addition, and react for 60 minutes. After the reaction was completed, 8 g of sodium metabisulfite was added and stirred for 15 minutes. Add 25ml of 80% acetic acid and heat up to -65°C. Add 300 ml of 10% sodium chloride aqueous solution, stir evenly after adding, and measure the cephalothin residue ≤ 5% by HPLC.
[0082] Heat th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com