Method for synthesizing 2-aryl benzopyrone flavonoid derivatives
A synthesis method and technology of pyrone, which are applied in the fields of drug combination, organic chemistry, metabolic diseases, etc., can solve the problems of high operation difficulty, cumbersome post-processing, long reaction period, etc., and achieve simple post-processing, shortened reaction time, heating fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
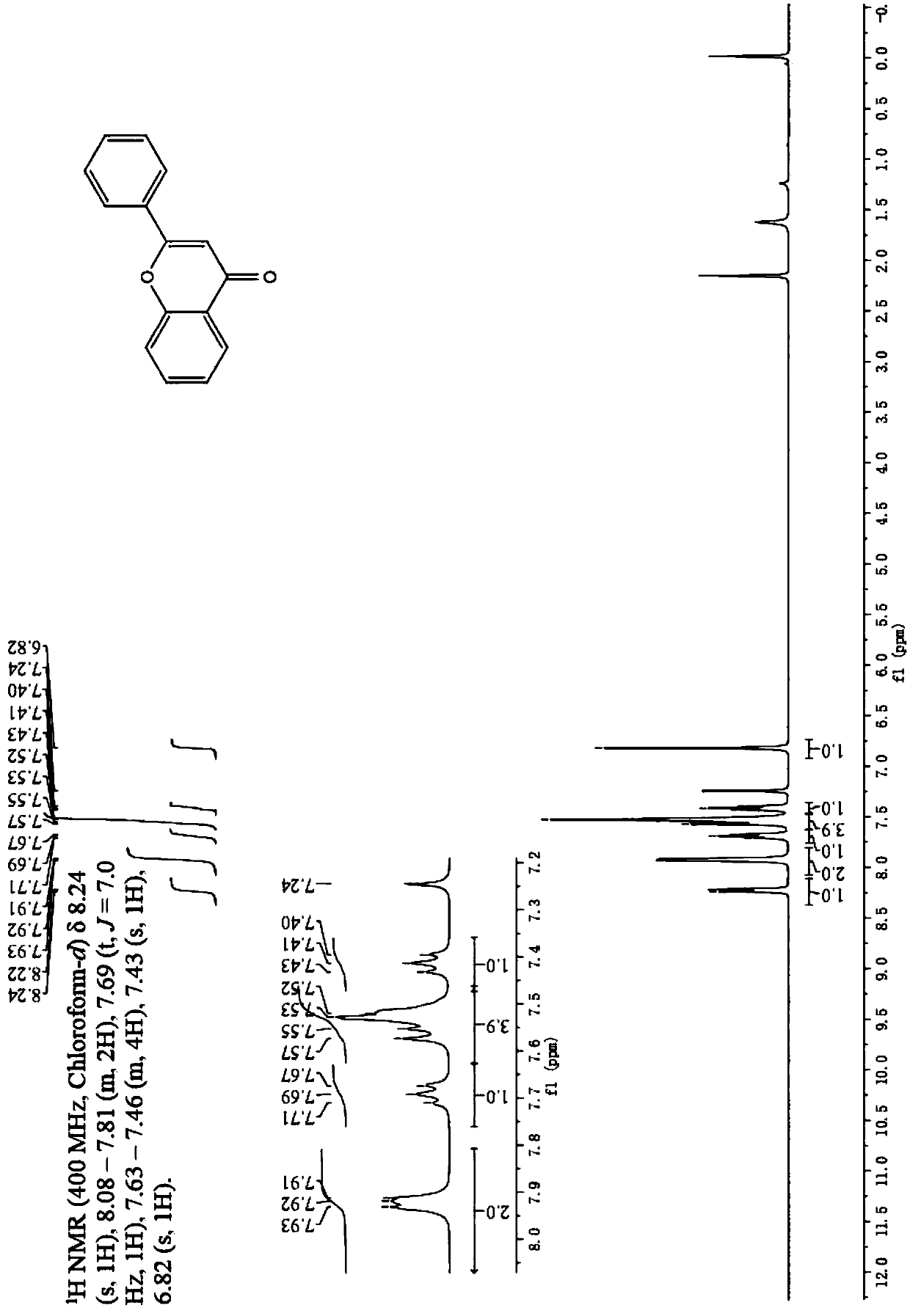
[0035] Embodiment 1: The synthesis method of 2-arylbenzopyrone flavonoid derivatives in this embodiment is carried out according to the following steps:
[0036] 1. In a microwave test tube, add 1.0mmol of aryl ethyl ketone, 10ml of organic solvent and 10ml of saturated alkaline aqueous solution in sequence, then dropwise add 1.2mmol of arylformyl chloride, and carry out microwave irradiation under the conditions of 60°C and microwave 100-150W. Irradiate for 5-6 minutes, then add 2.0 mg of phase transfer catalyst and 5 ml of saturated alkali solution, and then irradiate with microwave for 3-4 minutes under the condition of microwave 100-150W. Recrystallization to obtain yellow powder β-propanedione compound;
[0037] 2. Add the β-propanedione compound into the microwave test tube, then add 8ml of organic solvent, 1.02mmol of trifluoromethanesulfonic acid and CuCl 2110 mg was dissolved, and irradiated by microwave at 65°C and microwave 100-150W for 5-6min, poured the reaction ...
specific Embodiment approach 2
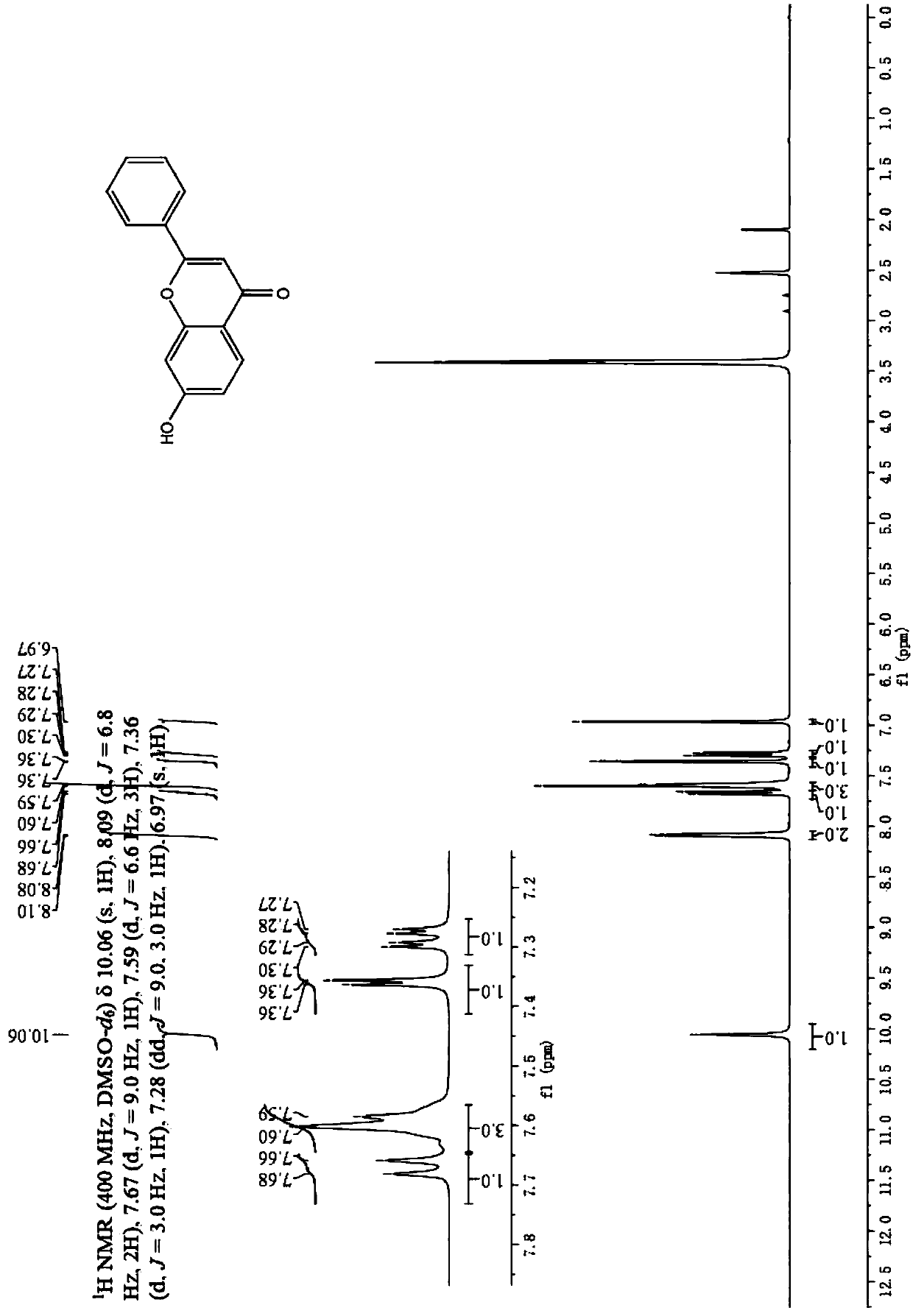
[0038] Specific embodiment 2: The difference between this embodiment and specific embodiment 1 is that the aryl ethyl ketone described in step 1 is o-hydroxyacetophenone, 2,4-dihydroxyacetophenone or 2-hydroxy-4-methoxy Acetophenone. Others are the same as in the first embodiment.
specific Embodiment approach 3
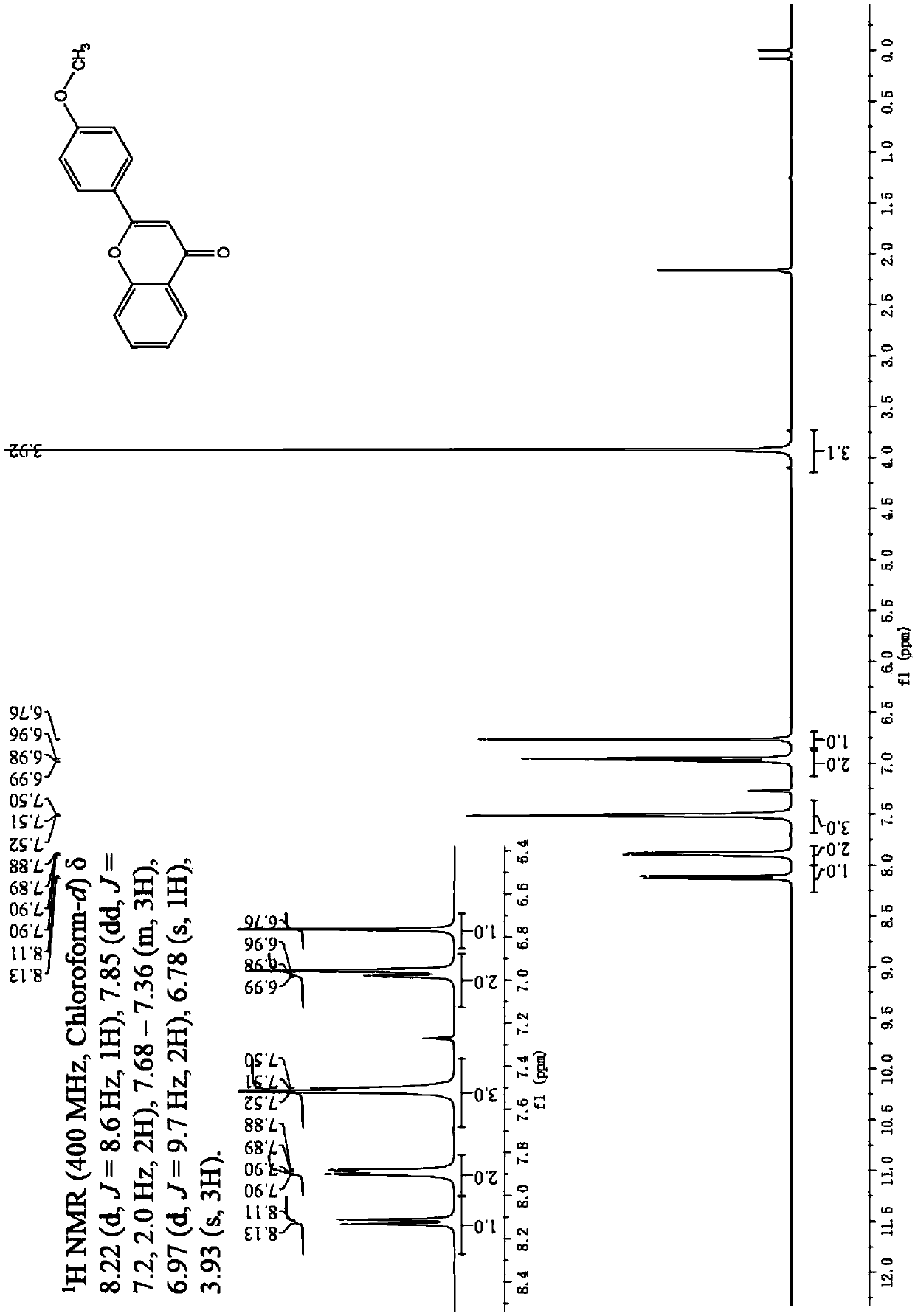
[0039] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that the aryl formyl chloride described in step 1 is benzoyl chloride, p-methoxybenzoyl chloride or p-chlorobenzoyl chloride. Others are the same as in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com