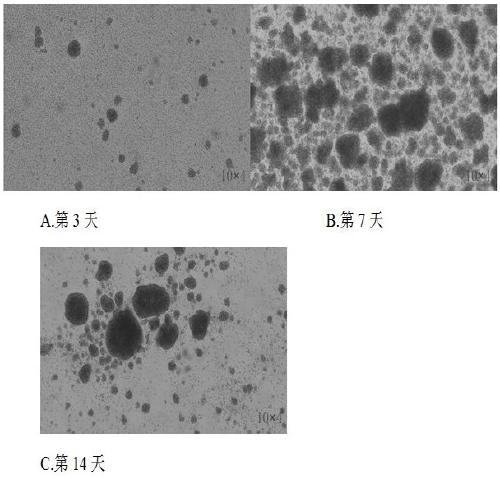
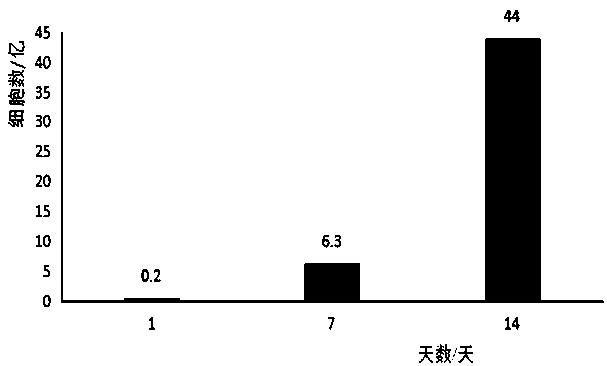
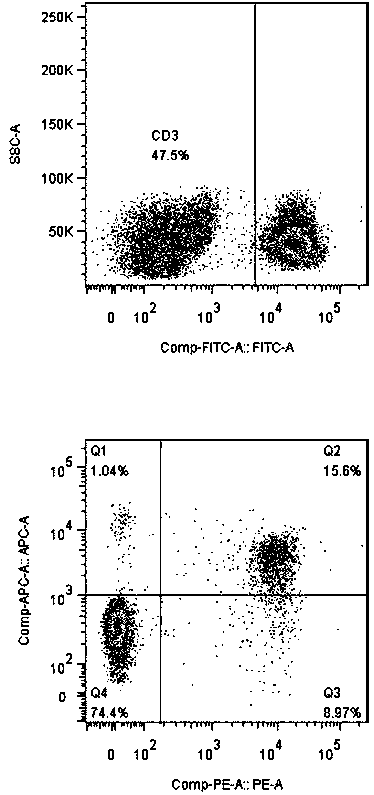
Method for in vitro multiplication culture of NK cells
An in vitro culture and NK cell technology, applied in the field of in vitro culture and expansion of NK cells, can solve the problems of instability, low amplification multiple and purity, and unverified safety, and achieve high safety and strong expansion ability. , the effect of simple amplification method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A method for culturing NK cells in vitro, after culture bottle Coating, peripheral blood collection, plasma inactivation, mononuclear cell separation, seed bottle, amplification, bottle transfer, cell harvesting and detection, the specific steps are as follows:
[0025] (1) Culture flask Coating: Prepare a mixed solution of heparin sodium, CD16 and human immunoglobulin, in which the content of heparin sodium is 500U / ml, the content of CD16 is 5ug / ml, and the content of human immunoglobulin is 1mg / ml. 5ml covered the bottom of non-TC-treated T75 culture flask, and placed the culture flask in a refrigerator at 4°C. After 12 hours, the liquid in the culture bottle was discarded, and the culture bottle was washed twice with PBS for later use.
[0026] (2) Peripheral blood collection: Volunteers tested negative for hepatitis B surface antigen, hepatitis C antibody, human immunodeficiency virus antibody, Treponema pallidum antibody, and cytomegalovirus IgM antibody. Take 50m...
Embodiment 2
[0034] (1) Coating of culture flasks: Cover the bottom of non-TC-treated T75 culture flasks with a coating solution containing sodium heparin, CD16 and human immunoglobulin, and place the culture flasks in a refrigerator at 4°C. After 10 hours, the liquid in the culture bottle was discarded, and the culture bottle was washed twice with PBS for the following operations. The content of heparin sodium in the coating solution is 500U / ml, the content of CD16 is 5ug / ml, and the content of human immunoglobulin is 1mg / ml.
[0035] (2) Peripheral blood collection: Volunteers tested negative for hepatitis B surface antigen, hepatitis C antibody, human immunodeficiency virus antibody, Treponema pallidum antibody, and cytomegalovirus IgM antibody. Take 50ml of peripheral blood, put it in a 10ml sterile sodium heparin blood collection tube, and separate it within 2 hours.
[0036] (3) Plasma inactivation: Put the peripheral blood into a centrifuge and centrifuge at 3000 rpm for 10 minutes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com