Preparation method of zero-valent iron sulfide and application thereof
A zero-valent iron and elemental sulfur technology, applied in the field of environmental chemistry, can solve the problems of zero-valent iron and sulfur waste, low utilization rate of sulfur, harsh vulcanization conditions, etc., achieve less energy consumption, facilitate large-scale promotion, The effect of short preparation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Take 0.014g of elemental sulfur powder and 0.246g of zero-valent iron in a 52mL serum bottle. Under anaerobic conditions, add 26 mL of 200 mM morpholineethanesulfonic acid (MES) solution with an initial pH of 6.00 to the serum bottle. After the serum bottle is sealed, it is placed in a rotary mixer for 12 hours of rotating and mixing reaction to obtain zero-valent iron sulfide. After filtering and freeze-drying, dry zero-valent iron sulfide is obtained.
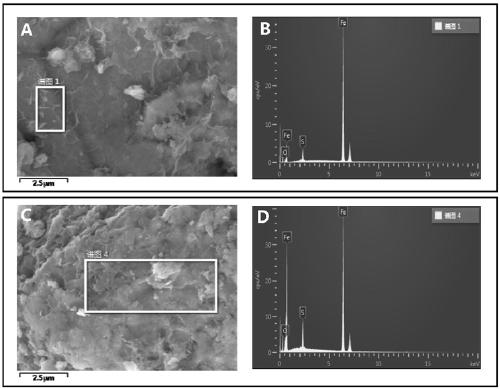
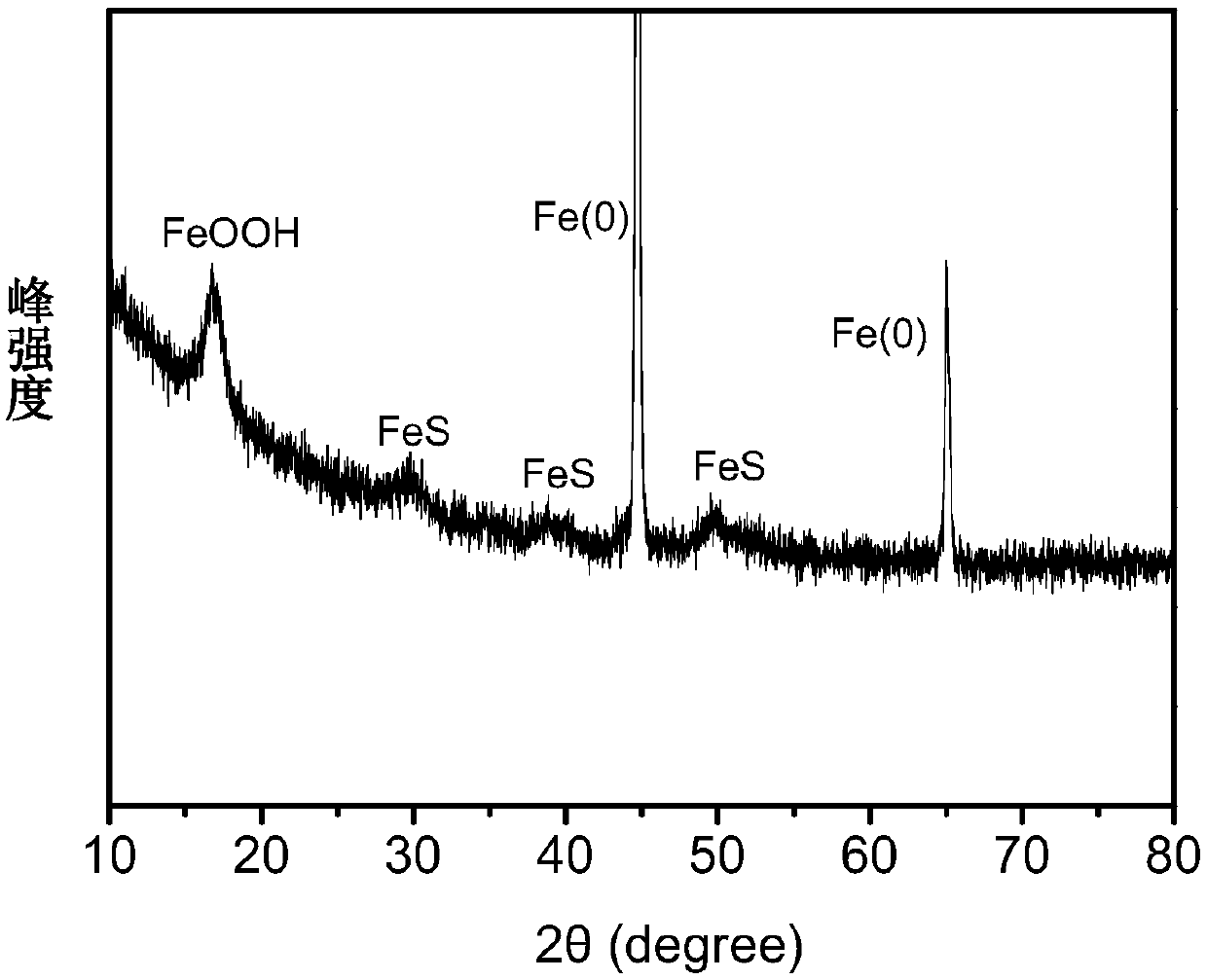
[0058] Figure 1~3 These are the SEM-EDS diagram, XRD diagram and XPS diagram of the zero-valent iron sulfide prepared in this embodiment in sequence. figure 1 can show the proportion of Fe, O, and S elements in the material particles, and we can clearly observe the existence of S elements in the material, indicating that the reaction of elemental sulfur and zero-valent iron can form sulfides on the surface of iron particles. At the same time, it can be observed that the distribution of S element on the particle su...
Embodiment 2
[0060] Take 0.014g of elemental sulfur powder and 0.246g of zero-valent iron in a 52mL serum bottle. In an anaerobic environment, add 26 mL of 10 mM CaCl with an initial pH of 4.00 to the serum bottle 2 aqueous solution. After the serum bottle is sealed, place it in a rotary mixer and mix and react for 12 hours to obtain zero-valent iron sulfide. After filtering and freeze-drying, dry zero-valent iron sulfide is obtained.
Embodiment 3
[0062] Take 0.014g of elemental sulfur powder and 0.246g of zero-valent iron in a 52mL serum bottle. In an anaerobic environment, add 26 mL of MgCl with an initial pH of 4.00 and 10 mM to the serum bottle 2 aqueous solution. After the serum bottle is sealed, place it in a rotary mixer and mix and react for 12 hours to obtain zero-valent iron sulfide. After filtering and freeze-drying, dry zero-valent iron sulfide is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com