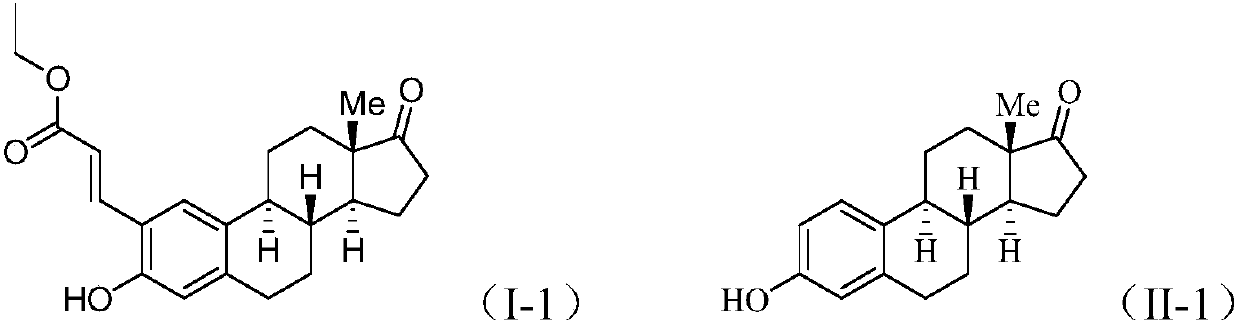Olefinated estrogen compound as well as preparation and application thereof
An estrogen and compound technology, applied in the field of olefinic estrogen compounds and their preparation, can solve the problems of low yield and the like, and achieve the effects of excellent inhibitory activity, novel structure and good antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: Preparation of olefinated estrogenone
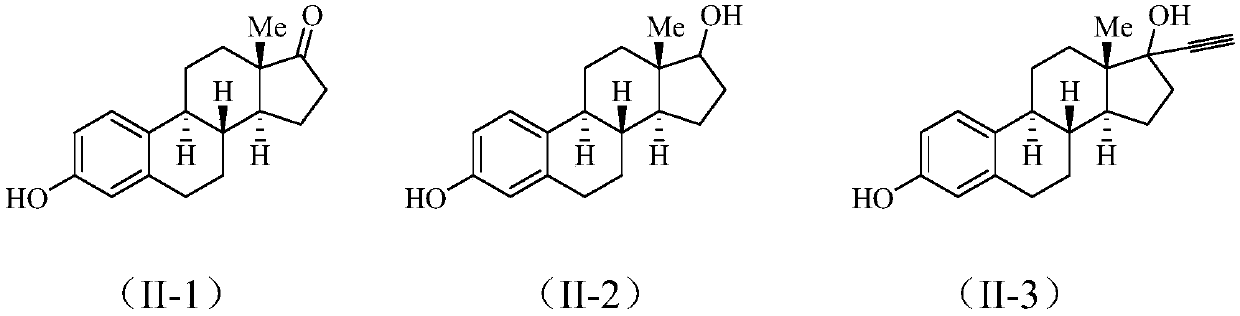
[0022] Add 1mmol estrone (II-1) to 4ml of acetic acid solvent, add 0.2mmol palladium acetylacetonate, 1.2mmol ethyl acrylate, 2.0mmol silver acetate, 2.0mmol potassium persulfate, and react at 80°C for 12 hours. After the reaction was completed, a saturated NaCl aqueous solution was added to the reaction liquid, extracted with dichloromethane, the organic layer was dried over anhydrous sodium sulfate, filtered, and evaporated to dryness under reduced pressure to obtain a crude compound. The crude compound was subjected to silica gel column chromatography, using a solution of ethyl acetate and petroleum ether with a volume ratio of 1:8 as the mobile phase, and the eluent with an Rf value of 0.3-0.5 was tracked and collected by TLC, and the collected eluent was passed through The solvent was removed under reduced pressure and dried to obtain 37 mg of a pure compound represented by formula (I-1).
[0023] Formula (I-1)...
Embodiment 2
[0025] Embodiment 2: Preparation of olefinated estradiol
[0026] Add 1mmol estradiol (II-2) to 4ml of acetic acid solvent, add 0.2mmol palladium acetylacetonate, 1.2mmol ethyl acrylate, 2.0mmol silver acetate, 2.0mmol potassium persulfate, and react at 80°C for 12 hours. After the reaction was completed, a saturated NaCl aqueous solution was added to the reaction liquid, extracted with dichloromethane, the organic layer was dried over anhydrous sodium sulfate, filtered, and evaporated to dryness under reduced pressure to obtain the crude compound (I-2). The crude compound (I-2) was subjected to silica gel column chromatography, using a solution with a volume ratio of ethyl acetate and petroleum ether of 1:5 as the mobile phase, followed by TLC to collect the eluent with an Rf value of 0.3-0.5, and obtained The eluate was desolventized under reduced pressure and dried to obtain 29 mg of pure compound represented by formula (I-2).
[0027] NMR data of the compound of formula (...
Embodiment 3
[0029] Embodiment 3: Preparation of olefinated estynol
[0030] Add 1mmol estynol (II-3) to 4ml of acetic acid solvent, add 0.2mmol palladium acetylacetonate, 1.2mmol ethyl acrylate, 2.0mmol silver acetate, 2.0mmol potassium persulfate, react at 80°C for 12 hours, After the reaction, a saturated NaCl aqueous solution was added to the reaction liquid, extracted with dichloromethane, the organic layer was dried over anhydrous sodium sulfate, filtered, and evaporated to dryness under reduced pressure to obtain the crude compound (I-3). The crude compound (I-3) was subjected to silica gel column chromatography, and a solution with a volume ratio of ethyl acetate and petroleum ether of 1:5 was used as the mobile phase, and the eluent with an Rf value of 0.3-0.5 was tracked and collected by TLC to obtain The eluate was desolventized under reduced pressure and dried to obtain 29 mg of pure compound represented by formula (I-3).
[0031] NMR data of the compound of formula (I-3): 1H ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com