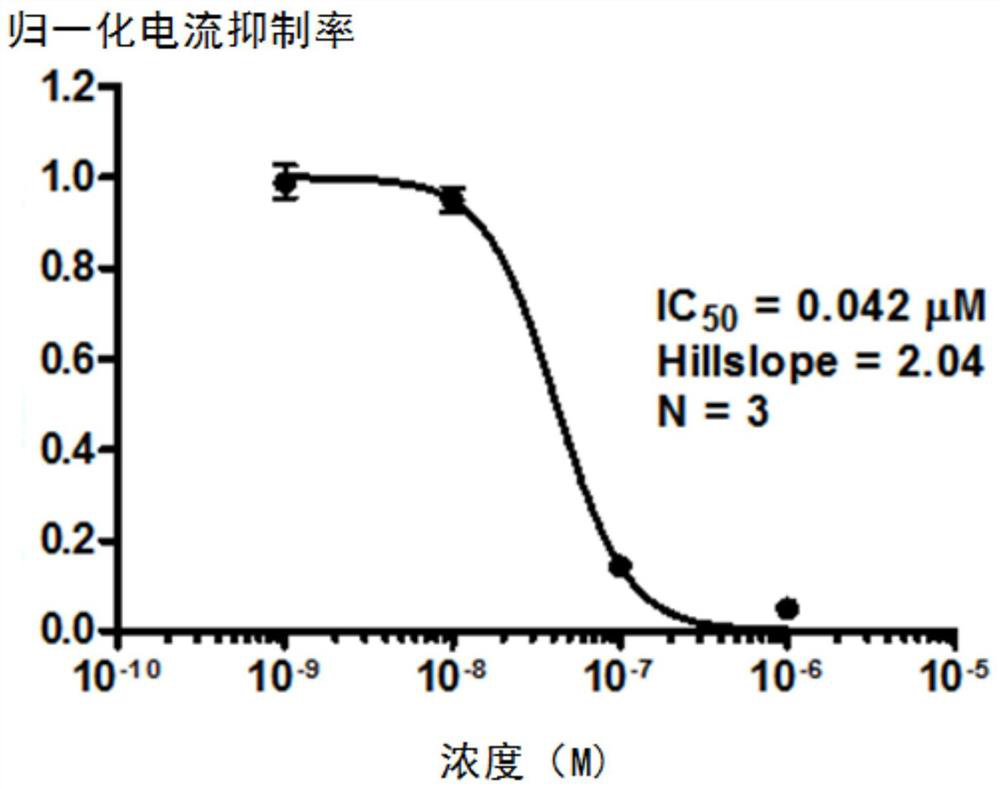
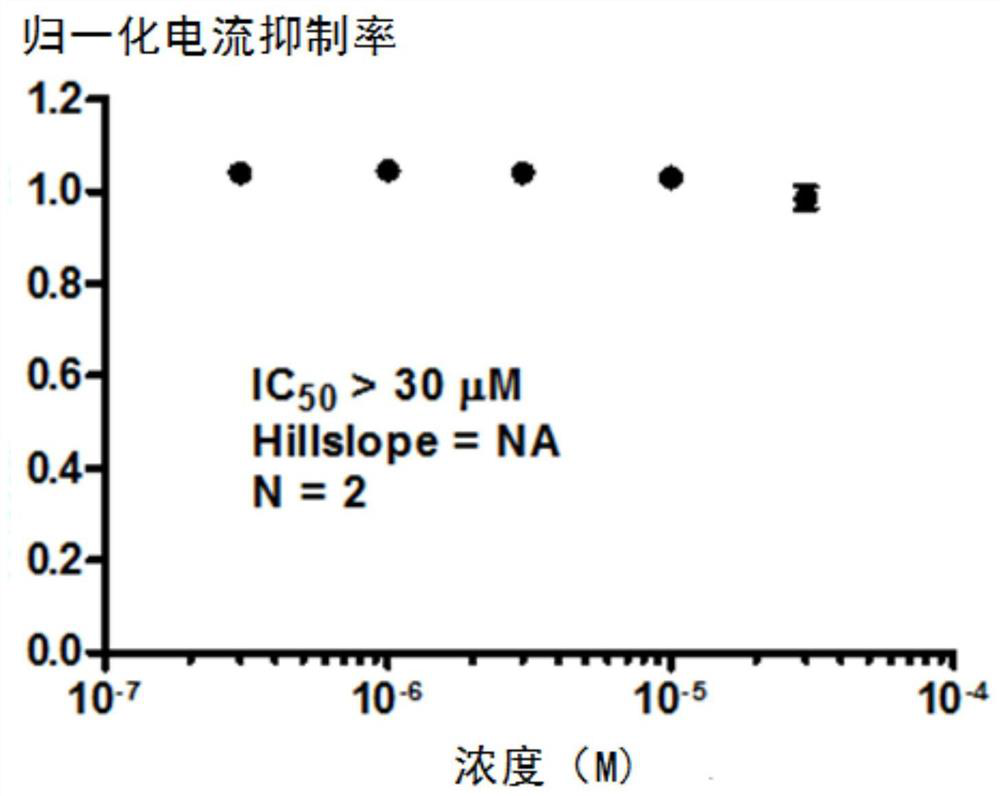
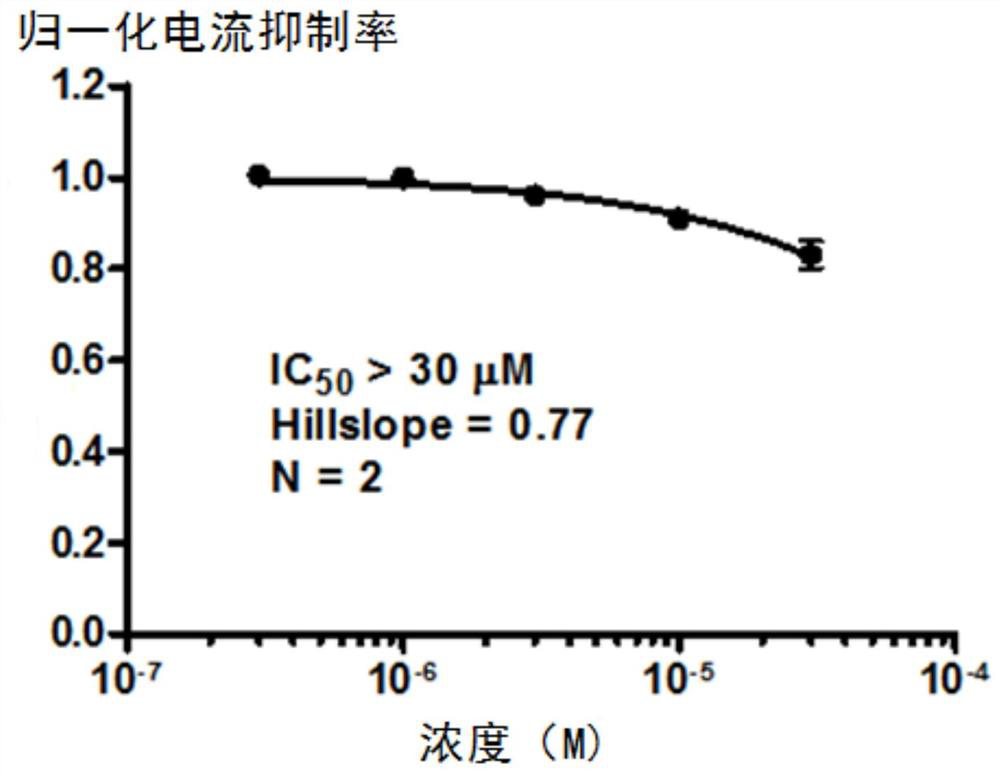
Substituted urea compound, its pharmaceutically acceptable salt or its solvate, its application, medicine and pharmaceutical composition
A compound, the technology of replacing urea, applied in the direction of drug combination, organic chemistry, medical preparations containing active ingredients, etc., can solve the problem that the tumor suppressing effect needs to be improved, and achieve prolonging the time of drug resistance, broad application prospects, and improving curative effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The substituted urea compound described in the embodiment of the present invention can be prepared by various methods, and the embodiment of the present invention provides a method for preparing the substituted urea compound with a higher yield, which includes the following steps:
[0053] (1) compound (Ic) is obtained by reacting compounds (Ia) and (Ib);
[0054]
[0055] (2) obtain compound (Ie) by reaction of compound (Ic) and (Id);
[0056]
[0057] (3) obtaining the substituted urea compound by reacting compound (Ie) and compound (If);
[0058]
[0059] Among them, R 1 selected from methoxy or deuterated methoxy;
[0060] R 2 selected from methyl, deuterated methyl, any structure in
[0061] R 3 Any one selected from F, Cl, Br, I.
[0062] Compound (Ia) and Compound (Ib) are pharmaceutical intermediates and can be purchased. In step (1), compound (Ia) and compound (Ib) are amidated under basic catalytic conditions to obtain compound (Ic). Used ba...
Embodiment 1
[0083] The synthetic route of compound I-1 1-(2-chloro-4-((6,7-dimethoxyquinazolin-4-yl)oxy)phenyl)-3-cyclopropylurea is as follows:
[0084]
[0085] resolve resolution:
[0086] (1) Add 4-amino-2-chloro-phenol (12.7g, 0.1mol) and sodium bicarbonate (16.8g, 0.2mol) to tetrahydrofuran (100ml) and water (100ml), stir at room temperature to make it Uniform suspension state (solution A). Phenyl chloroformate (18.8 g, 0.12 mol) was dissolved in tetrahydrofuran (50 mL) to form a solution (Solution B). Add solution B dropwise to solution A, stir the reaction at room temperature after the dropwise addition, track the reaction by TLC, analyze and confirm the completion of the reaction, and perform post-treatment. The post-processing steps include: standing the reaction solution for liquid separation, extracting the aqueous phase with 100 mL of ethyl acetate, washing the organic phase with saturated brine three times, adding anhydrous sodium sulfate to dry, suction filtering, and ...
Embodiment 2
[0091] The synthetic route of compound I-2 1-(2-fluoro-4-((6,7-dimethoxyquinazolin-4-yl)oxy)phenyl)-3-cyclopropylurea is as follows:
[0092]
[0093] The synthetic method can refer to Example 1, the equivalent ratio and reaction conditions in step (1) and step (2) are all constant, the reactant equivalent in step (3) is constant, and the reaction condition is 40 ℃ heating and stirring reaction. The post-treatment steps include: adding 100 mL of water to the reaction solution, crystallizing at room temperature under stirring, suction filtration, and washing the filter cake twice with purified water. The resulting solid was slurried in ethanol (200 mL), suction filtered, and vacuum-dried to obtain 6.05 g of compound (I-2), with a yield of 76%.
[0094] The NMR data of the obtained compound (I-2) are: 1H NMR (400MHz, DMSO) δ (ppm): 8.62 (s, 1H), 8.26 (d, 1H), 8.03 (s, 1H), 7.62 (s, 1H),7.55(d,1H),7.43(s,1H),7.32(d,1H),7.20(d,1H),4.12-3.96(m,6H),2.70-2.60(m,1H),0.73 (q, 2H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com