Lithium-sulfur battery positive copolymer sulfur material and prepared lithium-sulfur battery made from material
A lithium-sulfur battery and copolymerization technology, which is applied in the battery cathode material and battery field to achieve the effects of convenient operation, favorable for large-scale production, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Add 0.5g valeronitrile to 4.5g sublimated sulfur powder, and carry out low-speed ball milling to it for 4h, (the ball milling speed is 400r / min, every ten minutes interval of ball milling is 3 minutes) to obtain a stable homogeneous mixture;
[0041] (2) Heat the mixture obtained in step (1) to 120° C. at a heating rate of 10° C. / min.
[0042] (3) The mixture obtained in step (2) was kept at 120° C. and stirred for 6 h.
[0043] (4) The mixture obtained in step (3) was cooled to room temperature and then ball milled for 4 hours (the ball milling speed was 400 r / min, and the milling interval was 3 minutes every 10 minutes).
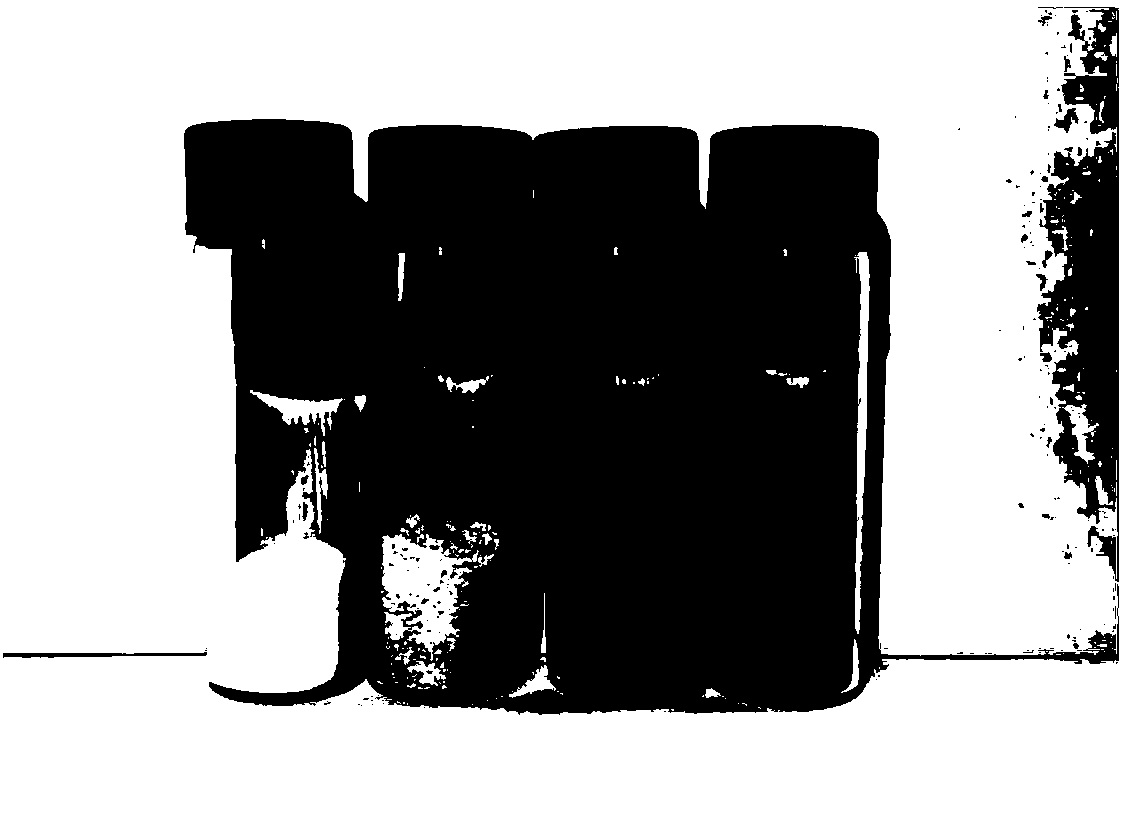
[0044] For pictures of sublimated sulfur powder see figure 1 The leftmost sample, the second from the left is the photo of the copolymerized sulfur product in which valeronitrile made in this embodiment accounts for 10%. figure 2 It is an electron micrograph of the copolymerized sulfur product, and it can be seen that the particle size of the...
Embodiment 2
[0049] (1) Add 1g of valeronitrile to 4g of sublimated sulfur powder, and carry out low-speed ball milling to it for 4h to obtain a stable homogeneous mixture;
[0050] (2) Heat the mixture obtained in step (1) to 120° C. at a heating rate of 10° C. / min.
[0051] (3) The mixture obtained in step (2) was kept at 120° C. and stirred for 6 h.
[0052] (4) The mixture obtained in step (3) was cooled to room temperature and ball milled for 4 hours.
[0053] figure 1 The third sample from the left is a photo of the copolymerized sulfur product in which valeronitrile produced in this example accounts for 20%. image 3 It is an electron micrograph of the copolymerized sulfur product. It can be seen from the figure that the copolymerized sulfur is in an agglomerated state, with a large particle size and a small amount of granular protrusions on the surface. Figure 4 is the XRD pattern of the product. Figure 5 As a result of thermogravimetric analysis of the product obtained in th...
Embodiment 3
[0056] (1) Add 1.5g valeronitrile to 3.5g sublimated sulfur powder, and carry out low-speed ball milling to it for 4h at a ball milling speed of 400r / min to obtain a stable homogeneous mixture;
[0057] (2) Heat the mixture obtained in step (1) to 120° C. at a heating rate of 10° C. / min.
[0058] (3) The mixture obtained in step (2) was kept at 120° C. and stirred for 6 h.
[0059] (4) The mixture obtained in step (3) was cooled to room temperature and ball milled for 4 hours.
[0060] figure 1 The rightmost sample is a photo of the copolymerized sulfur product in which valeronitrile produced in this embodiment accounts for 30%. Figure 4 is the XRD pattern of the product. Figure 5 As a result of the thermogravimetric analysis experiment on the product obtained in this example, the polymerized sulfur samples of 10wt.%, 20wt.% and 30wt.% valeronitrile at 0-650°C began to lose weight at about 130°C, which basically guaranteed the battery preparation process. thermal stabili...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com