Preparation method of antibacterial peptide gene and encoding peptide and prokaryotic expression thereof Pomfret brandt
A prokaryotic expression and antimicrobial peptide technology, which is applied in the field of aquatic animal immunity, can solve the problems of strong bacterial drug resistance and crisis ecological security, and achieve the effect of easy degradation and drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
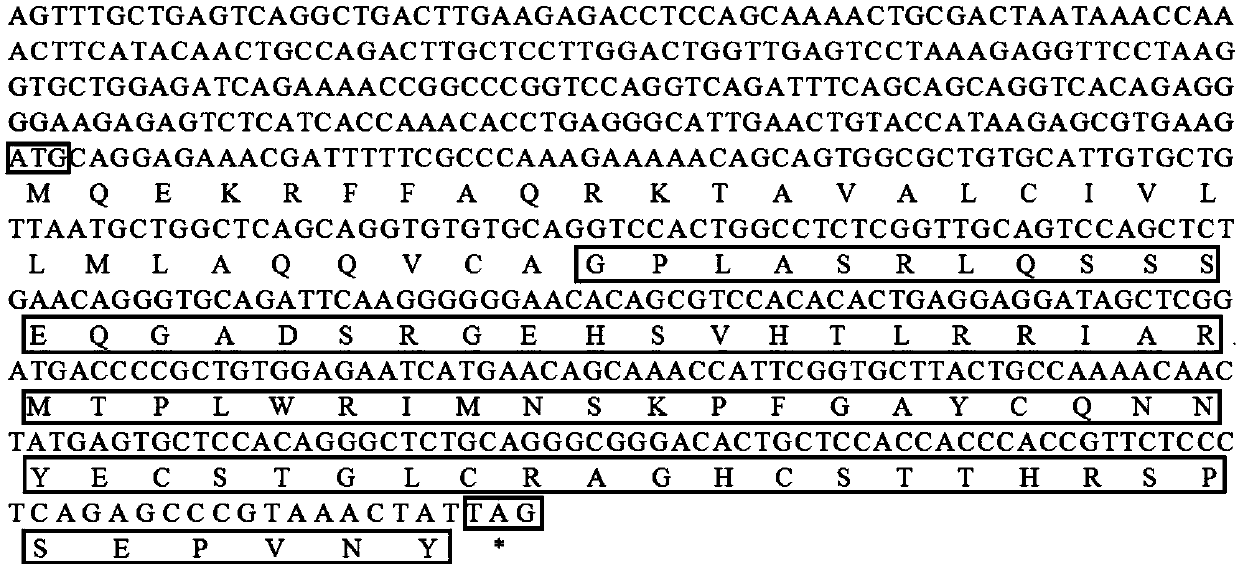
[0038] Example 1 The preparation method of the antimicrobial peptide gene and its coded peptide and prokaryotic expression of Bradley's pomfret comprises the following steps:
[0039] 1) Cloning and purification of the TroLEAP-2 fragment of the antimicrobial peptide gene of the pomfret pomfret
[0040] ①Extract liver RNA of Pompano brucei, reverse transcribe to obtain the cDNA library of Pomfret brucei, and use LEAP-2F1 / R1 primers to amplify to obtain the full-length sequence of the antimicrobial peptide gene TroLEAP-2 of Pomfret brucei without signal peptide, the specific sequence See SEQ NO.1.
[0041] Among them, the upstream primer LEAP-2F1:
[0042] 5'-AATTGATATCGCCACCATGGGTCCACTGGCCTCTC-3'
[0043] Downstream primer LEAP-2R1:
[0044] 5'-CTAAATTGATATCATAGTTTACGGGCTCTGAGG-3'
[0045] ②Gel recovery kit was used to purify the product, and the purified TroLEAP-2 fragment of the antimicrobial peptide gene of Pomfret pomfret without signal peptide was obtained.
[0046]③ ...
Embodiment 2
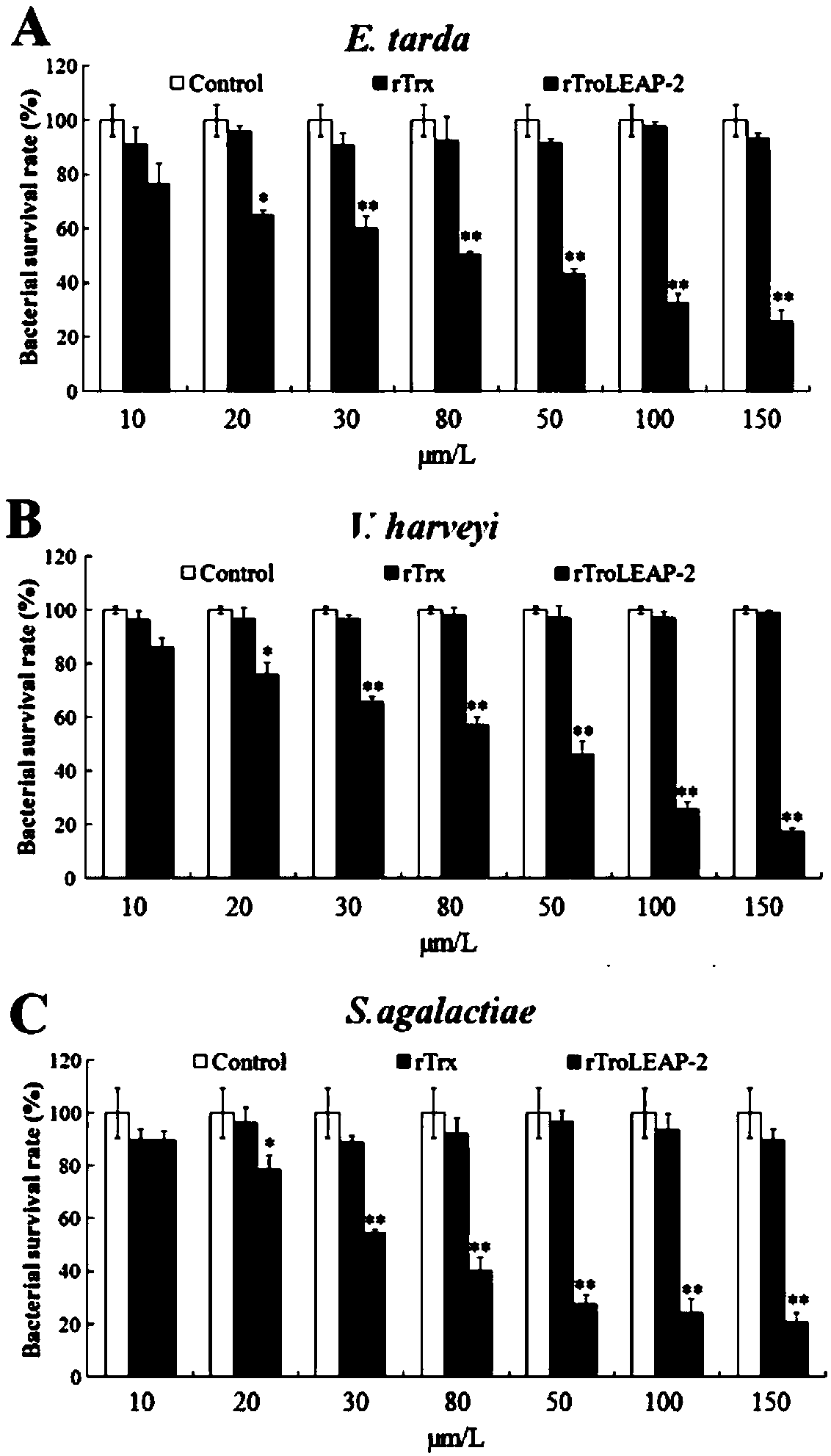
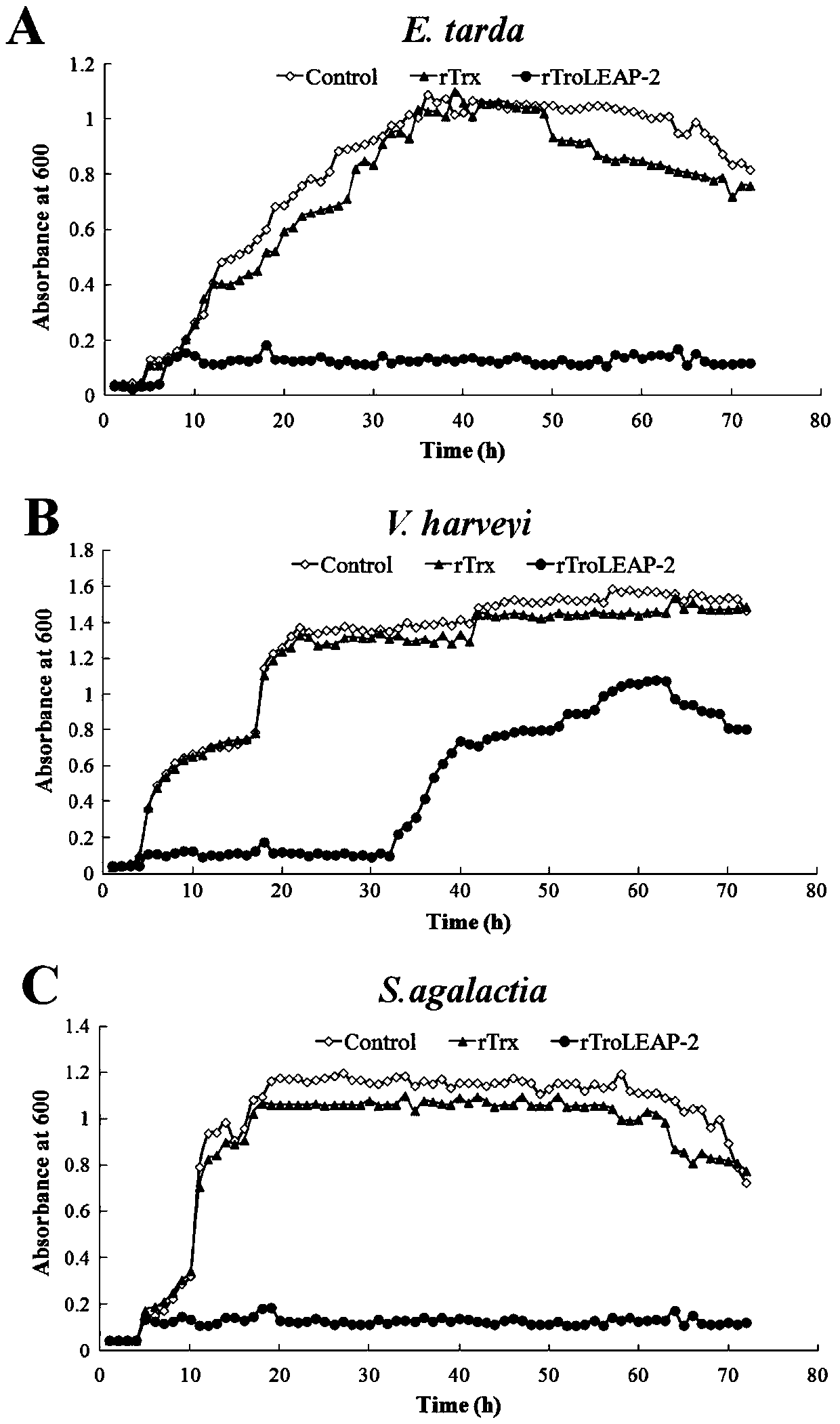
[0063] Example 2: In vitro antibacterial activity experiment of recombinant pompano antibacterial peptide
[0064] (1) Colony counting method
[0065] ①Dilute the three pathogenic bacteria grown to the logarithmic phase of Edwardsiella tarda, Vibrio harveii and Streptococcus agalactiae to 1×10 with LB medium. 5 CFU / mL.
[0066] ② Dilute the recombinant Bradley pomfret antimicrobial peptide (rTroLEAP-2 for short) and the prokaryotic expression empty carrier protein rTrx with PBS to 20 μg / mL, 40 μg / mL, 60 μg / mL, 80 μg / mL, 100 μg / mL, 200 μg / mL, 300 μg / mL, in addition, 100 μL PBS as a negative control.
[0067] ③ 100 μL of diluted pathogenic bacteria and 100 μL of proteins diluted to different concentrations were incubated at 28°C for 3 hours.
[0068] ④ After incubation, dilute the incubation solution 100 times, and take 100 μL for coating. Incubate overnight at 28°C.
[0069] ⑤Record the number of single colonies on each plate, the calculation formula is:
[0070] Bacteria...
Embodiment 3
[0078] Example 3: In vivo antibacterial activity experiment of recombinant pompano antimicrobial peptide rTroLEAP-2
[0079] (1) The purified recombinant TroLEAP-2 was resuspended in PBS to a final concentration of 200 μg / mL, and then mixed with an equal amount of aluminum and compound adjuvant. PBS was also mixed with the same amount of aluminum and compound adjuvant as a control group.
[0080] (2) 100 pomfrets weighing about 10 g were randomly divided into two groups, injected with 100 μl rTroLEAP-2 + aluminum and compound adjuvant and aluminum and compound adjuvant respectively, and cultured in a recirculating aquaculture system.
[0081] (3) Eight weeks after immunization, Edwardsiella lentus and Streptococcus agalactiae were used to challenge the virus respectively, and the cumulative mortality of pomfret in each group was observed and recorded. The relative protective effect RPS calculation formula is as follows:
[0082] RPS=(1-% cumulative mortality of sample group / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular mass | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com