A target of anti-tuberculosis mycobacterium and its application
A Mycobacterium tuberculosis and target technology, applied in the field of cell biology, can solve the problems of unclear molecular mechanism and decreased viability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Mycobacterium tuberculosis secretory protein PknG
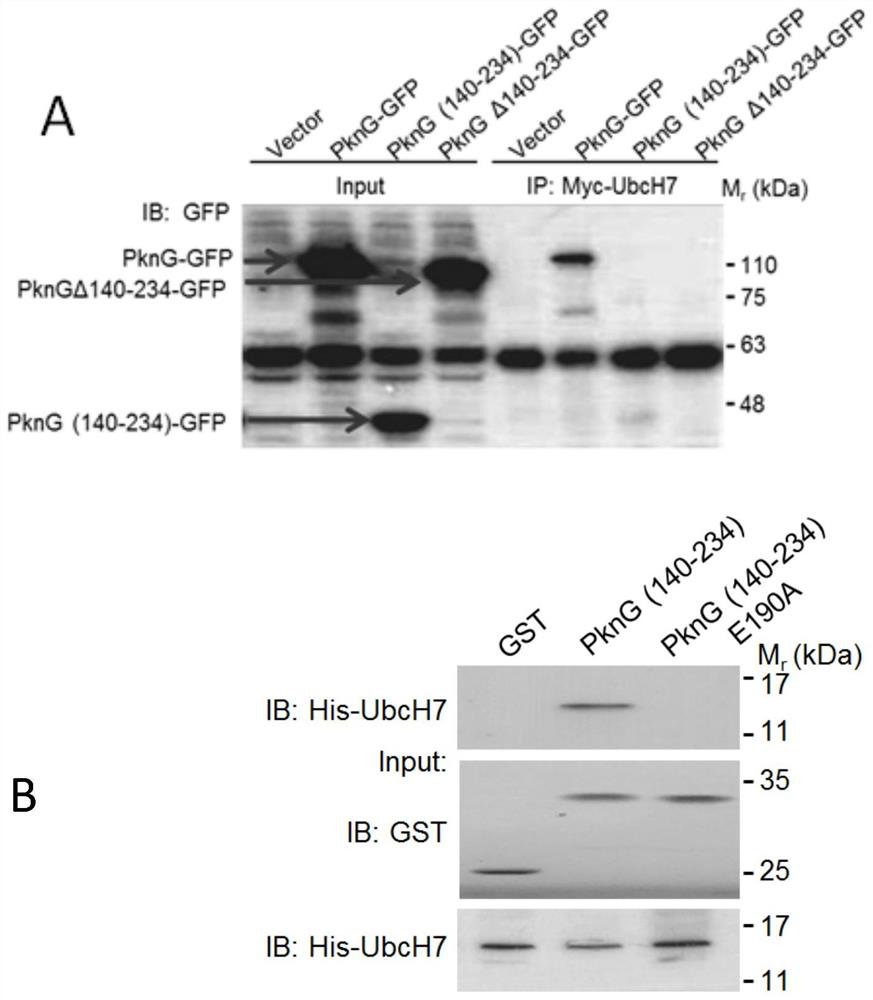
[0023] A ubiquitin-like domain, the amino acid sequence of which is shown in SEQ ID NO:1. This domain exists in Mycobacterium tuberculosis effector protein PknG (Gene ID: 886397 encoding) (amino acid sequence 140-234), which can interact with ubiquitin-conjugating enzyme (E2) to promote the intracellular survival process of Mycobacterium tuberculosis .
Embodiment 2
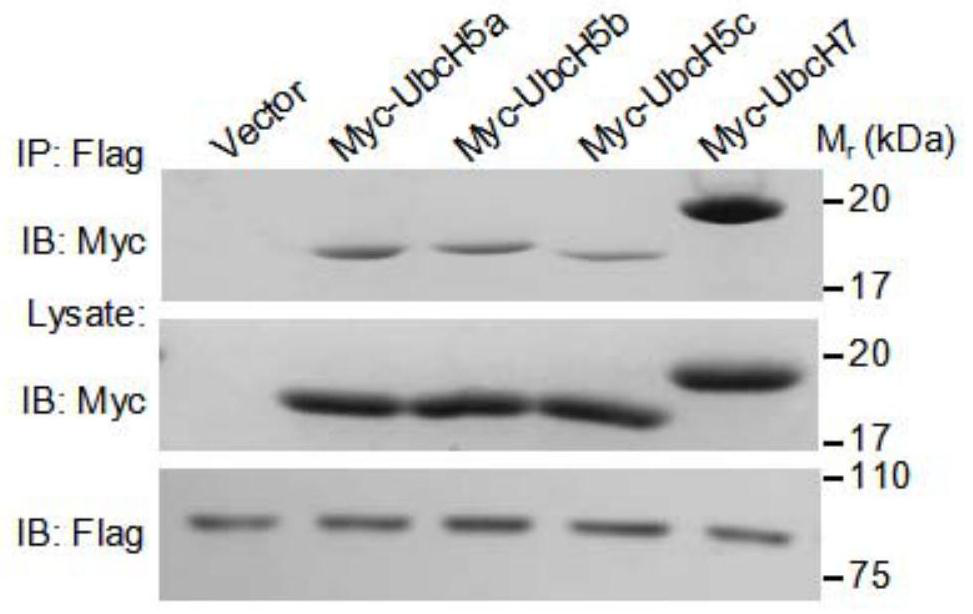
[0024] Example 2 PknG interacts with ubiquitin ligase (E2) through the ubiquitin-like domain
[0025] The E2s genes, including UbcH5a (Gene ID: 7321), UbcH5b (Gene ID: 7322), UbcH5c (Gene ID: 7323) and UbcH7 (Gene ID: 7332), were cloned into pcDNA6A respectively, and PknG and its truncated gene (including the gene encoding the ubiquitin-like domain and the gene of the PknG△(140-234) protein) were constructed into p3xflag CMV14 and pEGFP-N1 plasmids, respectively. The above-mentioned PcDNA6A plasmid containing the E2 gene was co-transfected with p3xflag CMV14-PknG into HEK293T cells, and the cells were placed in a 5% carbon dioxide incubator at 37 degrees Celsius for 24 hours; after 24 hours of transfection, the cell culture medium was aspirated, Wash the cells at least twice with PBS; add 1ml of cell lysate to fully lyse the cells on ice; transfer the cell lysate to a 1.5ml centrifuge tube, and centrifuge at 14,000rpm for 5 minutes at 4°C; transfer the supernatant to a new cen...
Embodiment 3
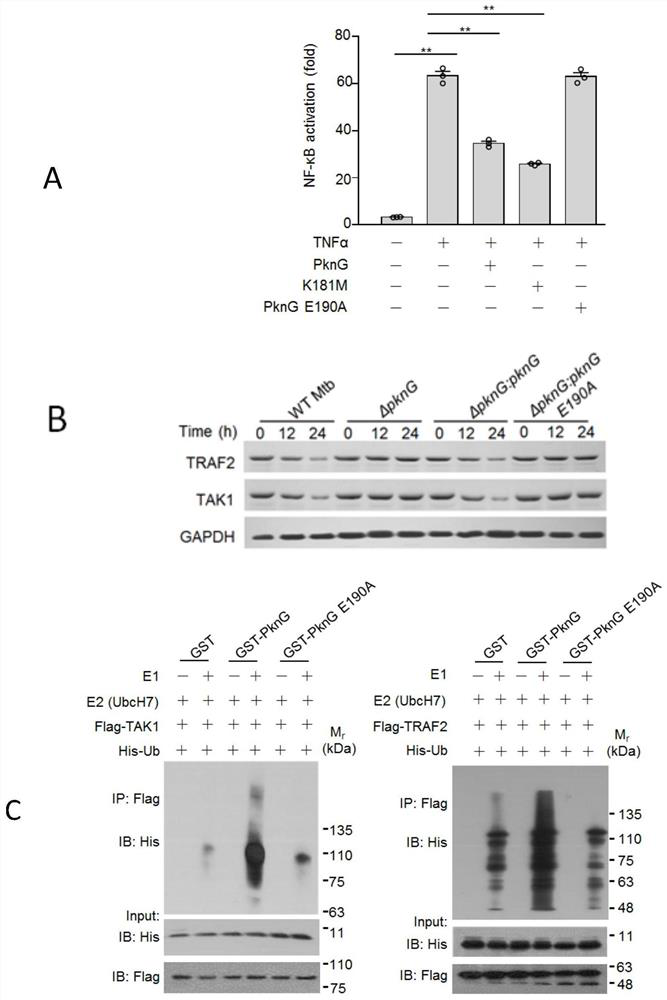
[0027] Example 3 PknG inhibits the activation of NF-κB innate immune signaling pathway by promoting the degradation of TRAF2 and TAK1, which depends on its ubiquitin-like domain
[0028]The dual-luciferase reporter gene system was used to detect the activation of NF-κB innate immune signaling pathway. The specific method was as follows: 1 μg pNF-κB-Luc and 50ng pRL-TK were transfected in HEK293T cells, and 1 μg PknG (p3xflag CMV14 -PknG) or the point mutant PknG K181M with loss of kinase activity or the point mutant PknG E190A with loss of ability to interact with E2, cells transfected with p3xflag CMV14 empty vector were used as control; 20 hours after transfection, add 20ng / ml TNFα (Invitrogen) was cultured for 6 hours; the cells were lysed, and the activation of NF-κB innate immune signaling pathway was detected using a dual luciferase reporter system detection kit (Promega). The results showed that PknG can inhibit the activation ability of NF-κB innate immune signaling pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com