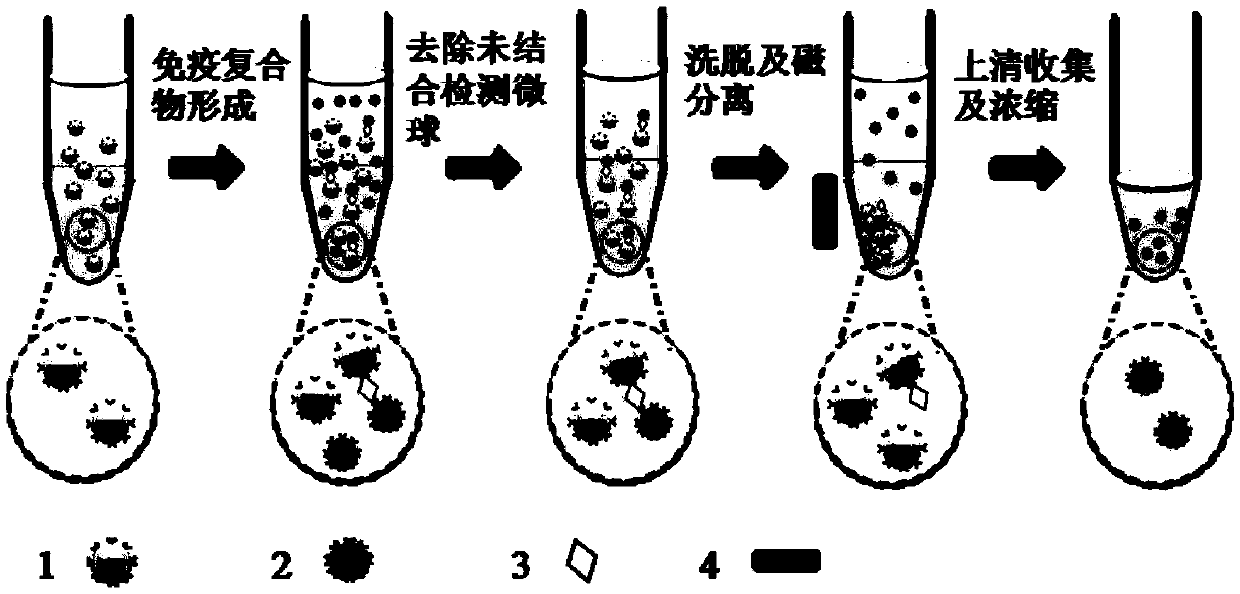
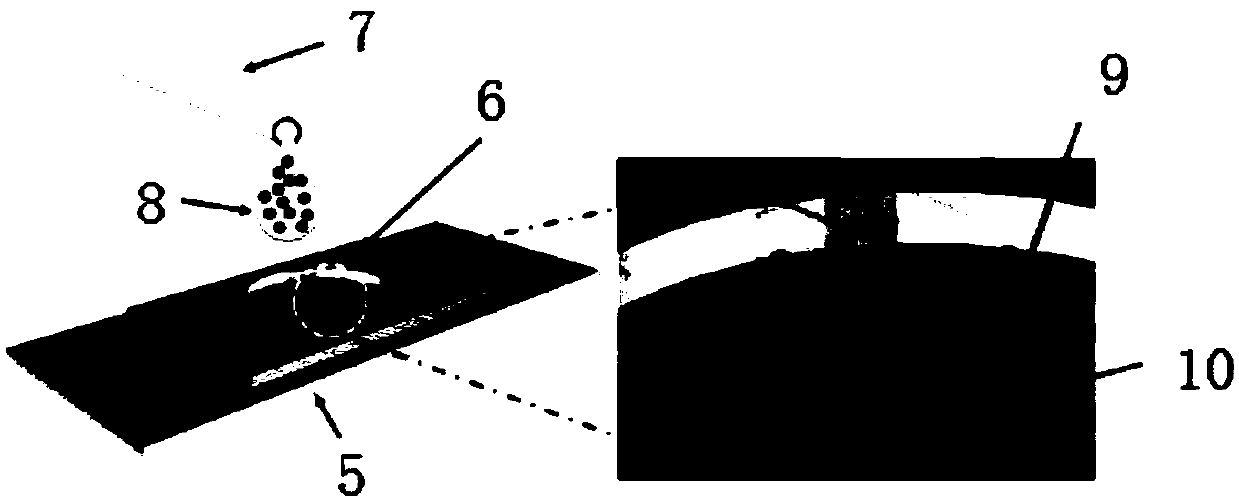
Low abundant protein absolute quantification method based on digital immunoassay technology
An immunoassay and absolute quantification technology, which is applied in the field of protein ultra-sensitive analysis, can solve problems such as inability to make absolute quantification, and achieve the effects of high accuracy, high throughput, and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Absolute quantitative analysis of CEA (carcinoembryonic antigen, carcinoembryonic antigen) standard
[0052] Preparation of magnetic capture beads: pipette 100 μL of magnetic beads (2×10 9 pcs / mL) stock solution in a 1mL centrifuge tube, washed twice with an equal amount of 0.01M NaOH solution, washed three times with an equal amount of deionized water, and then added 50 mg / mL EDC (i.e. 1-(3-dimethyl Aminopropyl)-3-ethylcarbodiimide hydrochloride) solution, incubate at room temperature for 30 minutes, remove the supernatant by magnetic separation, add 60 μL capture antibody solution (antibody mass is 120 μg), incubate at room temperature for 30 minutes , and then added 100 μL of PBS (phosphate buffer saline, phosphate buffered saline) buffer (pH=7.4) containing 0.1% tween20 to wash 4 times, and finally obtained capture magnetic beads, which were stored in a 4°C refrigerator for later use.
[0053] Preparation of detection particles: Pipette 100 μL of particle...
Embodiment 2
[0056] Example 2 Absolute Quantitative Analysis of Plasma CEA
[0057] The operating steps of embodiment 2 are the same as embodiment 1, only the CEA standard solution is replaced with diluted 10 5 times as many human plasma samples. The number of molecules of CEA in the plasma sample is equal to the number of detected particles, and the concentration of CEA can be calculated by combining the sample volume and Avogadro's constant. The results are shown in Table 1. Table 1 is a comparison table of the absolute quantitative analysis results of CEA in 10 plasma samples, the hospital test results and the standard addition analysis results. Among them, a is diluted 10 5 times the plasma, b is the whole plasma.
Embodiment 3
[0058] Example 3 The standard addition analysis of plasma CEA
[0059] Dilute #5 plasma sample by 10 5 times, take 5 parts of diluted plasma (100 μL each), add 0, 5, 10, 15, 20 μL of CEA standard solution with a concentration of 200 aM respectively, the final concentration of added CEA is 0, 10, 20, 30, 40 aM, Subsequent operation steps are the same as in Example 1, only the CEA standard solution needs to be replaced by the plasma sample added with CEA. Take the final concentration of CEA added in the plasma as the abscissa, and the number of detected particles as the ordinate, draw a standard curve (such as Figure 5 ). Figure 5 , the reverse extension line of the standard curve intersects the X-axis at a point, the abscissa of the intersection point is 37.3, diluted 10 5 Times the #5 plasma sample CEA concentration is 37.3aM. The concentration of CEA in #5 plasma stock solution was 0.57ng / mL. Under the same conditions, the standard addition curve of #10 plasma sample i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com