Preparation method of nickel boride base oxygen evolution catalyst
A technology of catalyst and nickel boride, which is applied in the field of electrochemical catalysis, can solve the problems of reduced electrocatalytic activity of materials, poor stability of electrocatalysts, and catalyst shedding, and achieves low cost, high electrochemical stability and activity, and large The effect of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
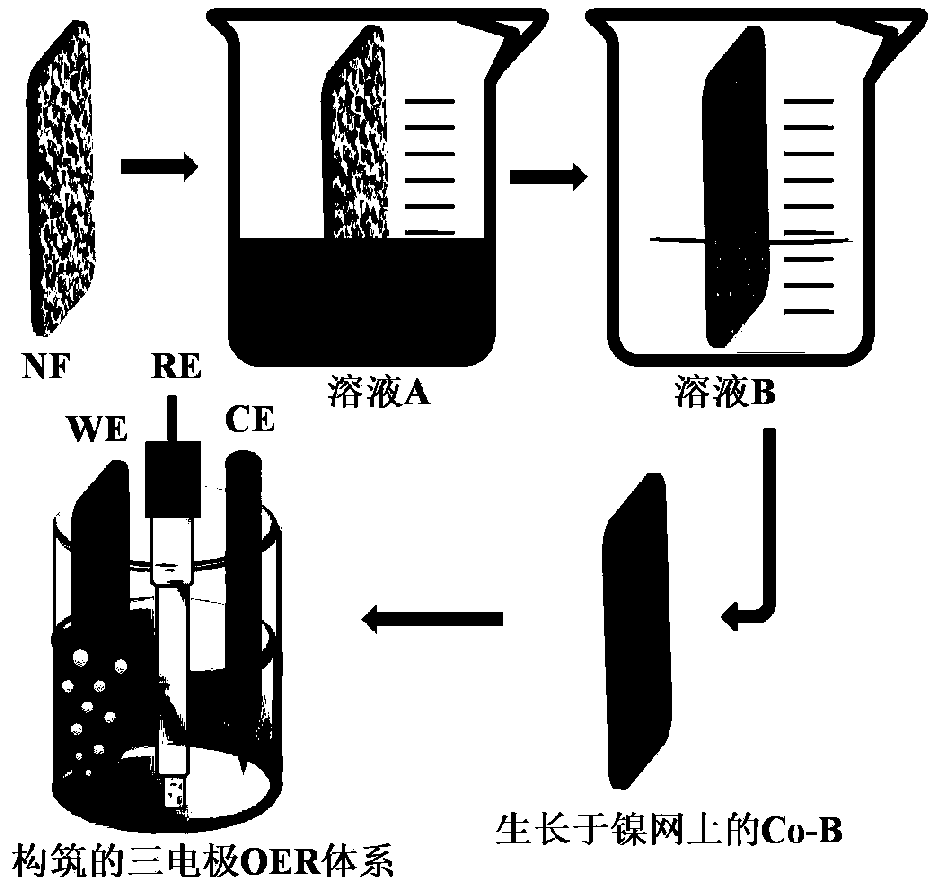
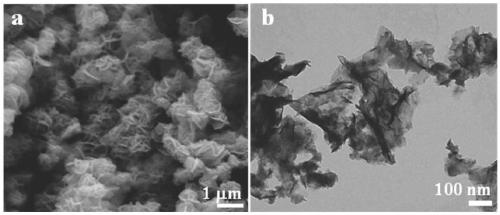
[0032] This embodiment relates to a preparation method of a nickel boride-based oxygen evolution catalyst, such as figure 1 As shown, it specifically includes the following steps:
[0033] Soak the nickel mesh used for the current-collecting base in dilute hydrochloric acid to remove the oxide layer, then use ethanol and double-distilled water to clean it ultrasonically, dry it under vacuum, and set aside;
[0034] Preparation concentration is the cobalt nitrate aqueous solution of 0.5mol / L and the sodium borohydride solution of 1mol / L dissolved in the sodium hydroxide solution of 0.1M respectively, and utilizes high-purity nitrogen to remove described cobalt nitrate aqueous solution and sodium borohydride respectively After the dissolved oxygen in the sodium hydroxide solution, set aside;
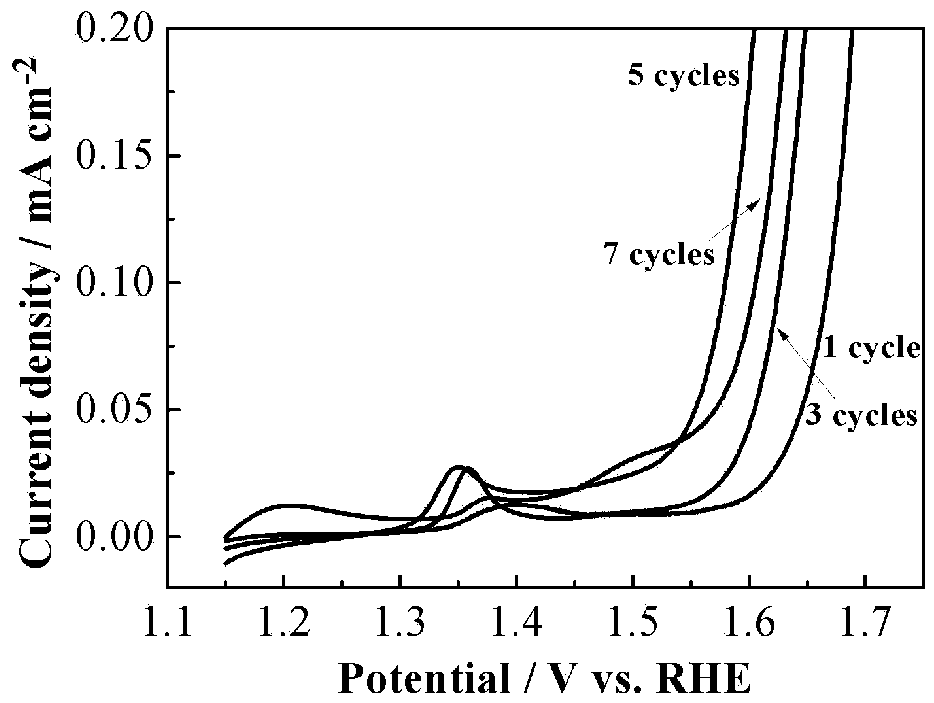
[0035] The nickel mesh is soaked successively in the sodium hydroxide solution of cobalt nitrate solution and sodium borohydride for 10 s, each soaking once in the sodium hydroxide soluti...
Embodiment 2
[0038] The only difference between this embodiment and Embodiment 1 is that the number of cycles is 1.
Embodiment 3
[0040] The only difference between this embodiment and embodiment 1 is that the number of cycles is 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com