Nucleic acid composition for detecting four enteroviruses simultaneously and kit and detection method
A nucleic acid composition, enterovirus technology, applied in the field of diagnosis, can solve problems such as unfavorable viruses, false positives, and mutual interference of primers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] A kit for the simultaneous detection of four enteroviruses is provided.
[0098] The kit includes RT-PCR reaction solution, nucleic acid composition for simultaneous detection of four enteroviruses, enzyme mixed solution, positive control and negative control.
[0099] Wherein, the RT-PCR reaction solution includes 250mM Tris-base, 0.25% TritonX-100, 25mmol / L MgCl 2 .
[0100] Nucleic acid compositions include:
[0101] The A6-type Coxsackievirus amplification primer pair whose sequences are shown in SEQ ID No.1 and SEQ ID No.2;
[0102] The A10-type Coxsackievirus amplification primer pair whose sequences are shown in SEQ ID No.3 and SEQ ID No.4;
[0103] The A16-type Coxsackievirus amplification primer pair whose sequences are shown in SEQ ID No.5 and SEQ ID No.6;
[0104] The EV71 enterovirus amplification primer pair whose sequences are shown in SEQ ID No.7 and SEQ ID No.8;
[0105] The A6-type Coxsackievirus detection probe whose sequence is shown in SEQ ID No...
Embodiment 2
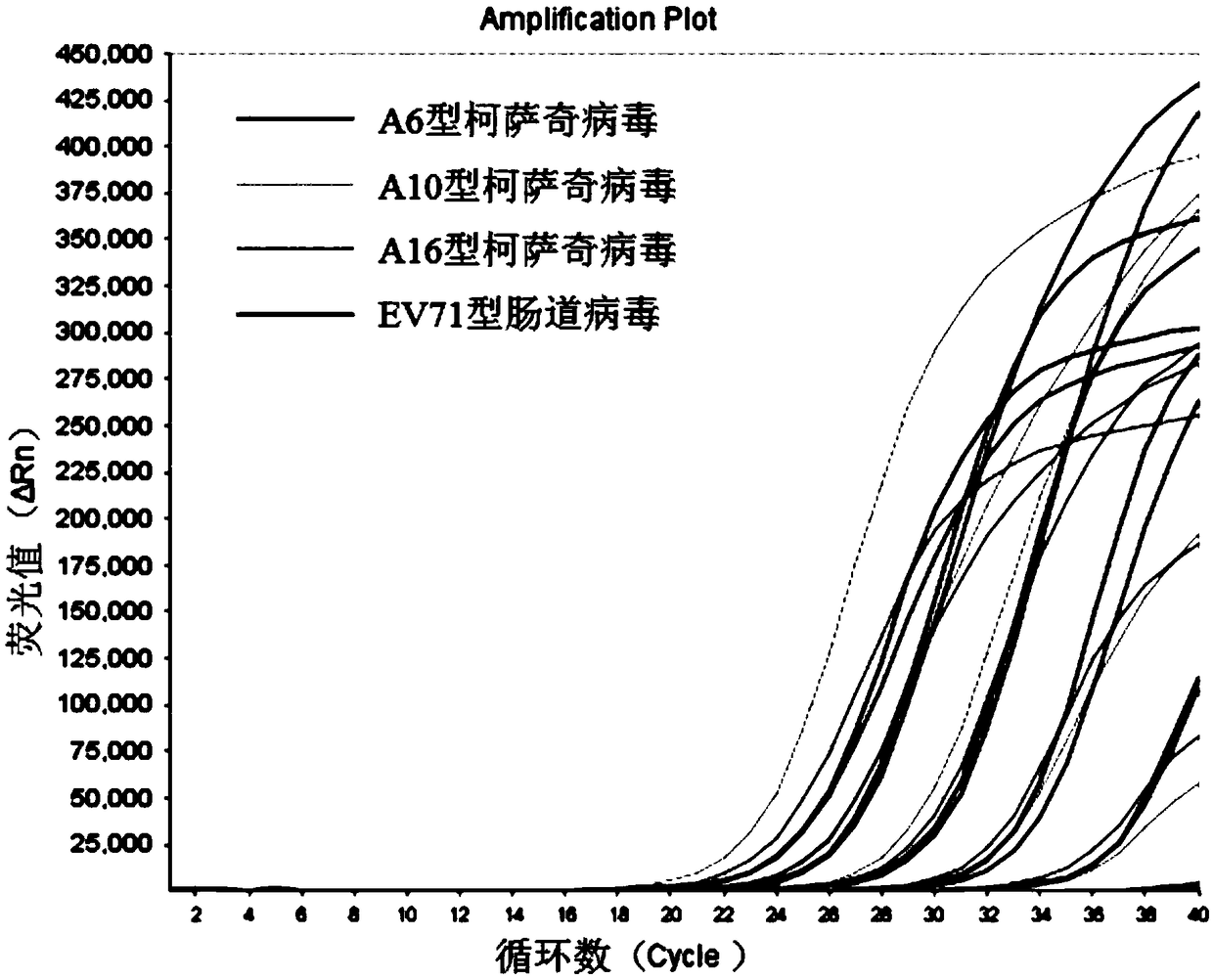
[0114] Measure the sensitivity of the test kit of four kinds of enteroviruses of simultaneous detection of embodiment 1
[0115] (1) A6 type Coxsackie virus positive standard, A10 type Coxsackie virus positive standard, A16 type Coxsackie virus positive standard, EV71 type enterovirus positive standard in the kit of embodiment 1 are mixed And dubbed a mixed solution to obtain a positive test article.
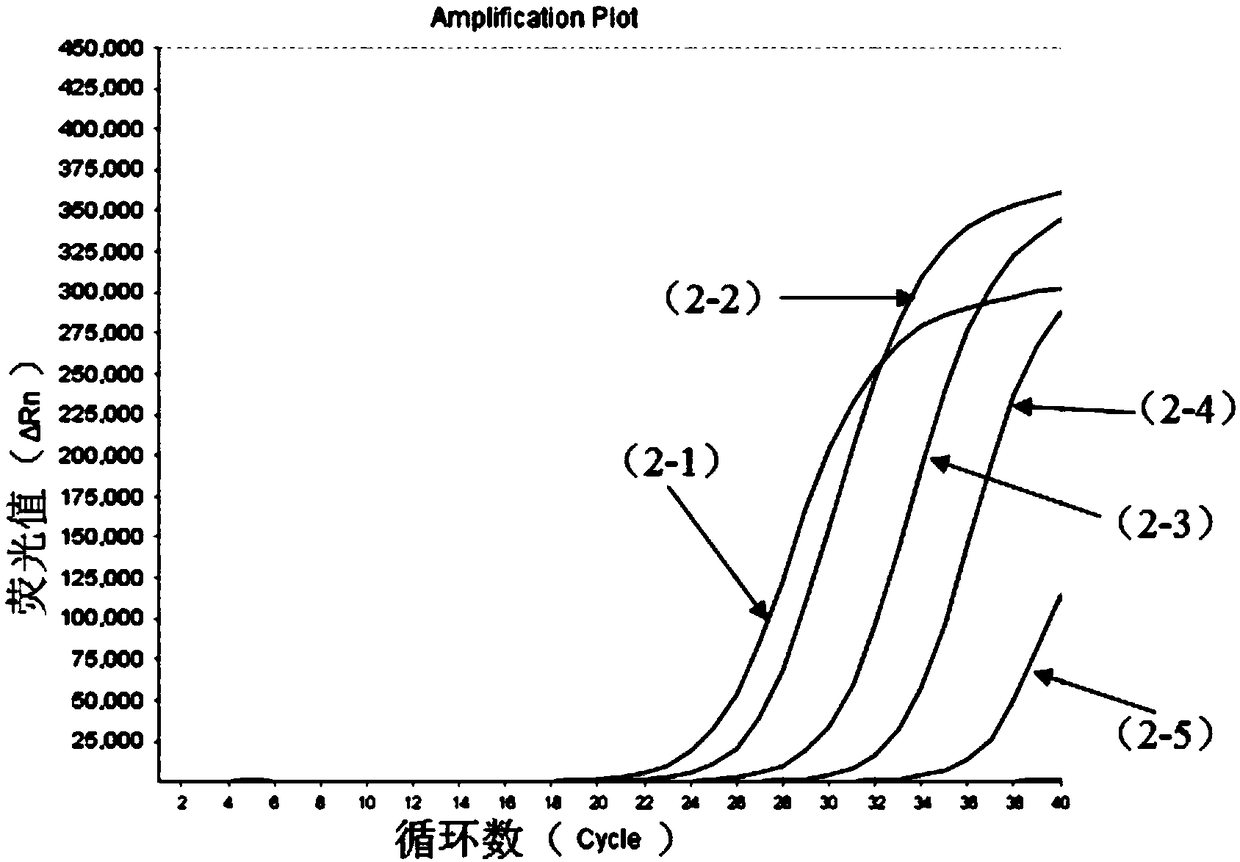
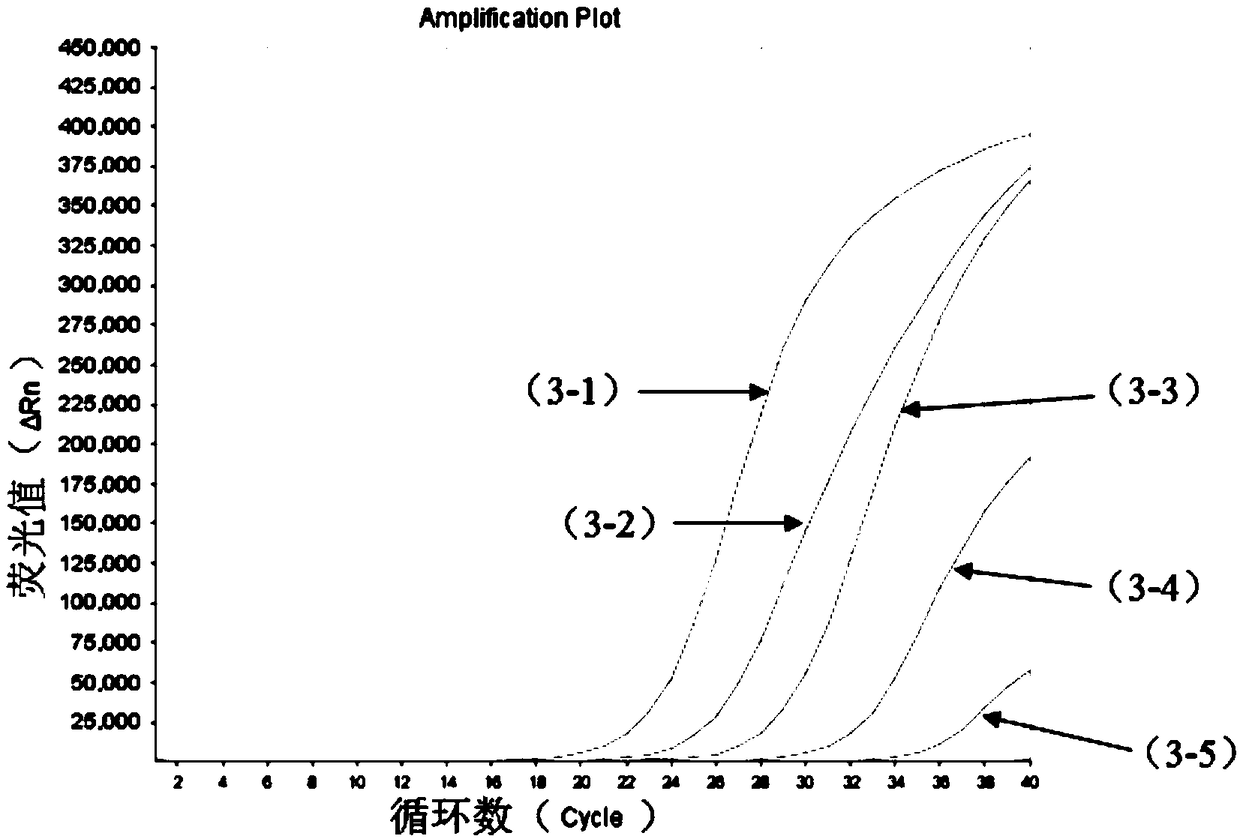
[0116] (2) Perform a 10-fold serial dilution of the positive sample to be tested (i.e. 10copies / mL~10 5 copies / mL), using the kit of Example 1 to perform multiple fluorescent quantitative PCR detection on the positive test items under each gradient, the system of multiple fluorescent quantitative PCR reactions is shown in Table 1. The reaction conditions of the multiplex fluorescent quantitative PCR reaction were: reverse transcription at 50°C for 15min; pre-denaturation at 95°C for 3min; denaturation at 95°C for 15s, annealing, extension, and signal acquisition at 55°C for 40s...
Embodiment 3
[0126] (1) A6 type Coxsackie virus positive standard, A10 type Coxsackie virus positive standard, A16 type Coxsackie virus positive standard, EV71 type enterovirus positive standard in the kit of embodiment 1 are mixed And make a mixed solution to obtain a positive test article; the normal saline not containing the above four enterovirus positive control substances is a negative test article.
[0127](2) The experiment is divided into an experimental group, a control group 1 and a control group 2, and the kit of the experimental group is the kit of Example 1. The kit of control group 1 is roughly the same as the kit of Example 1, except that the sequence of the EV71 enterovirus amplification primer pair is as shown in SEQ ID No.21 and SEQ ID No.22, and is compatible with EV71 The sequence of the EV71 type enterovirus detection probe corresponding to the type enterovirus amplification primer pair is shown in SEQ ID No.23. Specifically, the sequence shown in SEQ ID No.21 is: 5'...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com