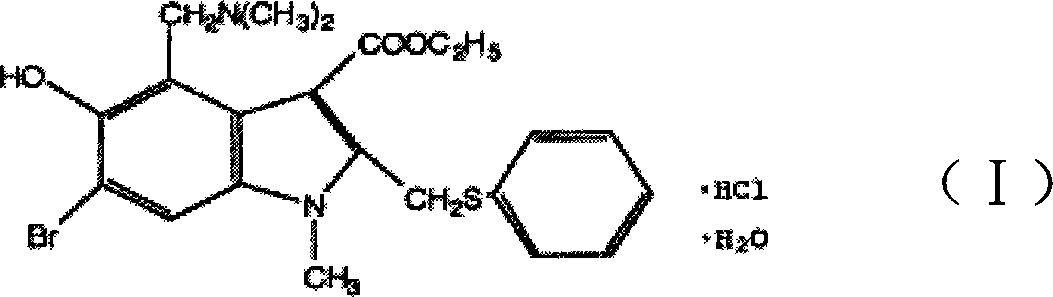
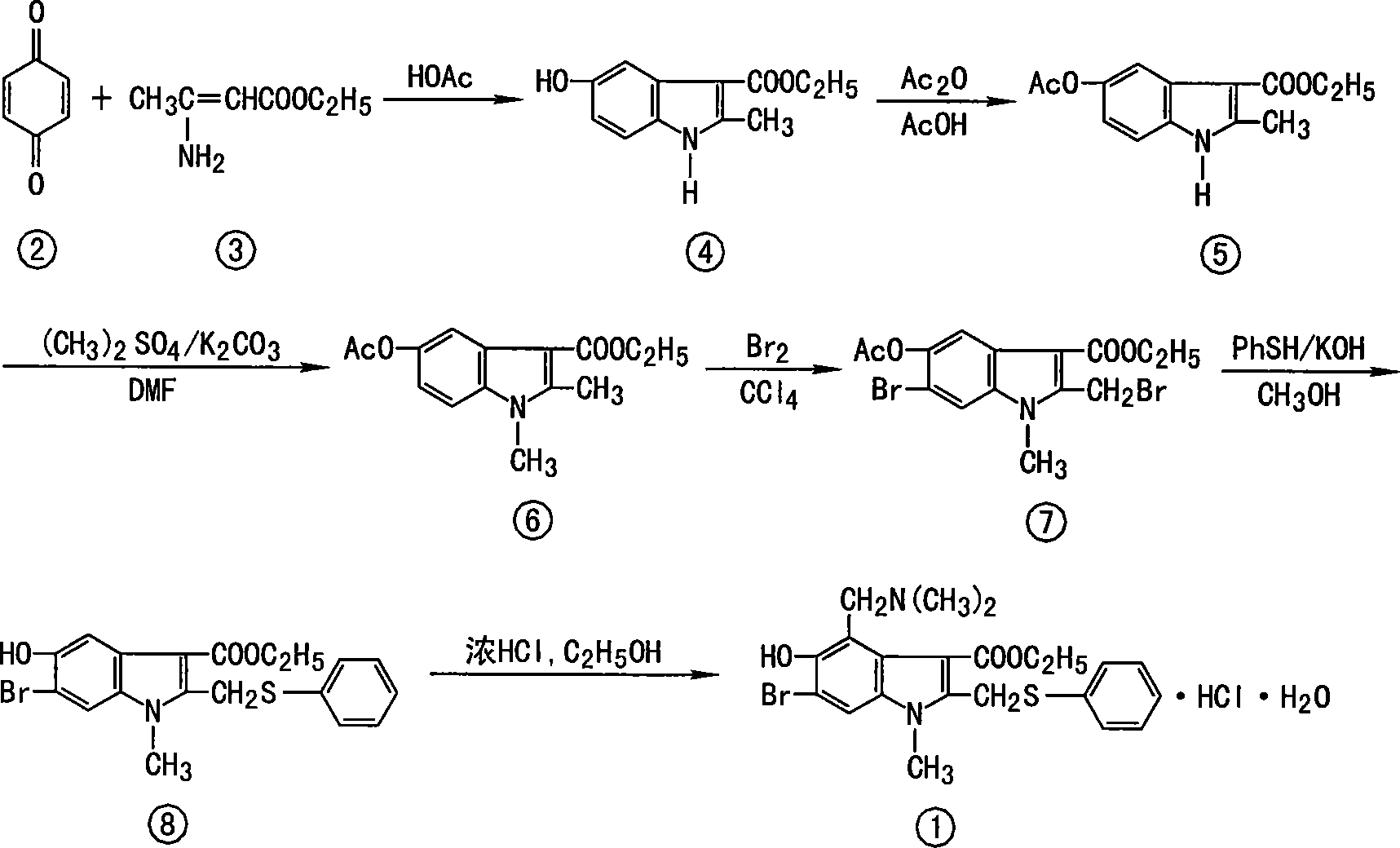
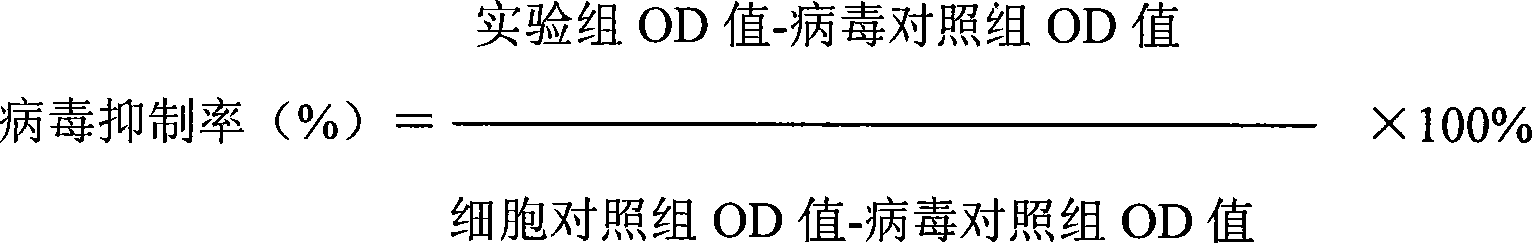Anti-respiratory virus medicine and use
A virus and drug technology, applied in the direction of antiviral agents, medical preparations containing active ingredients, pharmaceutical formulations, etc., to achieve the effects of good tolerance, convenient use and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: Arbidol hydrochloride is to the cytotoxicity of HEp-2 cell and HEL cell
[0023] Use trypsin to disperse well-grown HEp-2 cells and HEL cells into a single cell suspension, press 1×10 5 / ml concentration in 96-well plate, 0.1ml per well, set at 37°C, 5% CO 2 Cultivate in the incubator for 24h. After the cells grow into a single layer, the supernatant of the culture medium is discarded and replaced with a drug-containing maintenance solution of different concentrations. Four replicate wells are set for each concentration, and normal cells are set as a control. Continue culturing for 48 h, discard the culture supernatant, add 50 μl of serum-free MEM medium containing 5 mg / ml MTT (tetramethylazozolium salt) to each well, and place in CO 2 After continuing to culture in the incubator for 2-3 hours, discard the MTT supernatant, wash 3 times with PBS, add 100 μl of dissolving solution (DMSO:ethanol volume ratio: 1:1) to each well, shake for 5-10 minutes, and s...
Embodiment 2
[0033] Embodiment 2: Arbidol hydrochloride anti-Coxsackie virus effect in vitro
[0034] It is proposed that the effect of Arbidol hydrochloride on CVB3 is divided into four groups. Group I (drug antiviral biosynthesis group): first add virus liquid to adsorb cells for 2 hours, then add drug-containing maintenance liquid. Group II (drug direct action group): the virus solution reacted with different concentrations of drugs for 2 hours, and then the mixed solution was incubated with the cells for 2 hours, and then the cell maintenance solution was replaced. Groups III and IV (drug antiviral adsorption 2h, 8h groups): After adding different concentrations of drugs into the cells for 2h and 8h respectively, the drug solution was discarded, and the virus solution was added, and the cell maintenance solution was replaced after 2 hours of adsorption.
[0035]Four replicate wells were set in each well of the above four experimental groups, and uninfected cell control, virus control ...
Embodiment 3
[0044] Embodiment 3: Arbidol hydrochloride anti-adenovirus Ad7 effect in vitro
[0045] It is proposed that the effect of Arbidol hydrochloride on adenovirus Ad7 is divided into four groups. Group I (drug antiviral biosynthesis group): first add virus liquid to adsorb cells for 2 hours, then add drug-containing maintenance liquid. Group II (drug direct action group): the virus solution reacted with different concentrations of drugs for 2 hours, and then the mixed solution was incubated with the cells for 2 hours, and then the cell maintenance solution was replaced. Groups III, IV, and V (2h, 4h, and 8h groups of drug antiviral adsorption): add different concentrations of drugs into the cells for 2h, 4h, and 8h respectively, discard the drug solution, add virus solution, and absorb After 2 h, the cell maintenance solution was replaced.
[0046] Four replicate wells were set for each well of the above five groups of experimental groups, and uninfected cell control, virus contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com