Vanilline crystal form as well as preparation method and application thereof
A technology of vanillin and crystal form, which is applied in the field of vanillin crystal form and its preparation, can solve problems such as differences in drug dissolution and bioavailability, influence on drug absorption and utilization, and difference in drug solubility, so as to meet the requirements of bioavailability and drug solubility. Effectiveness requirements, avoiding changes in bioavailability and drug efficacy, and effects that are not easily affected by humidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Add 100g of vanillin, 630mL of water, and 63mL of ethanol into the reaction flask in sequence, raise the temperature to 75-85°C until clarification, remove insoluble matter by suction filtration while it is hot; heat the filtrate again until clarification, cool down to 10±5°C to crystallize for 6-8 Hour. Suction filtration, wash the filter cake (50mL×2). The filter cake was vacuum dried at 60°C to constant weight. 85.1 g of white crystals were obtained, the HPLC purity was 99.97%, and the yield was 85.1%.
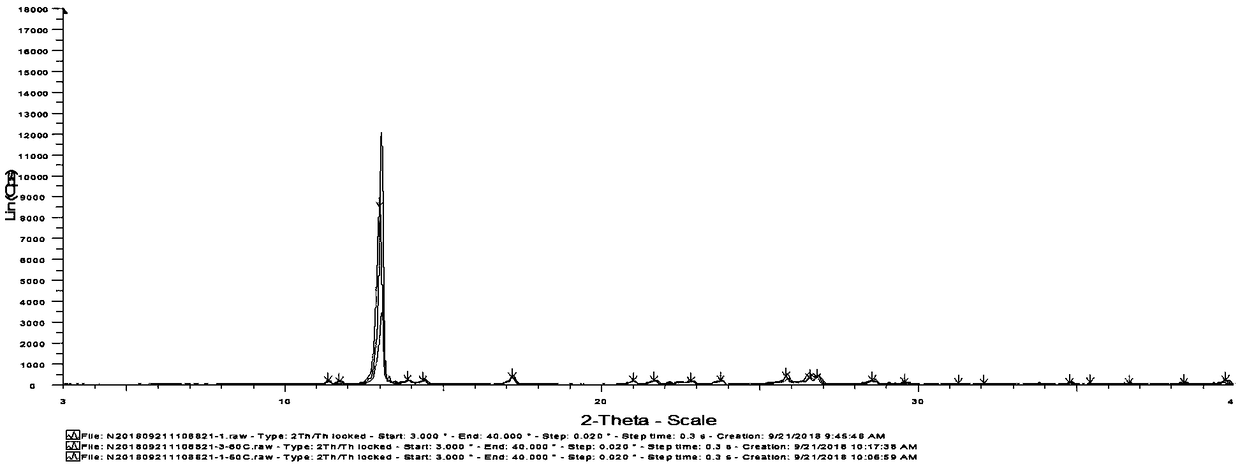
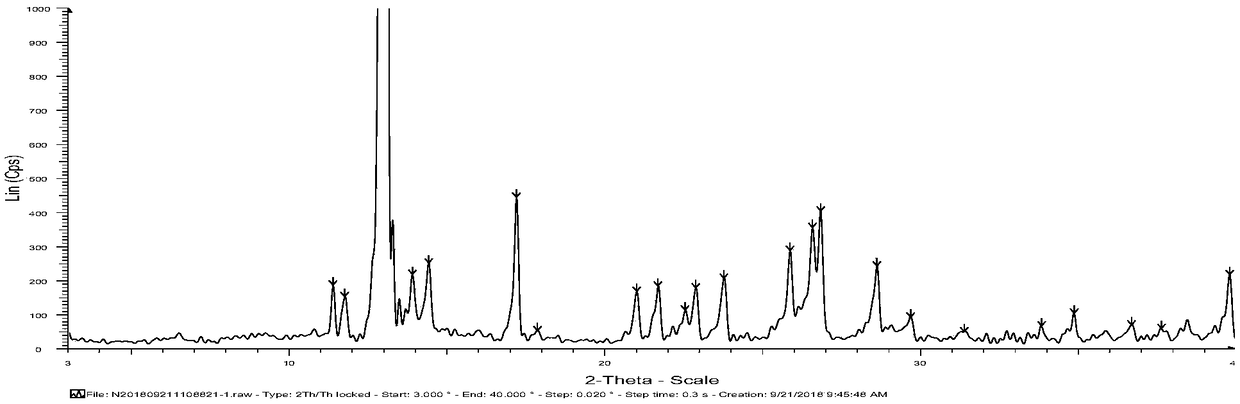
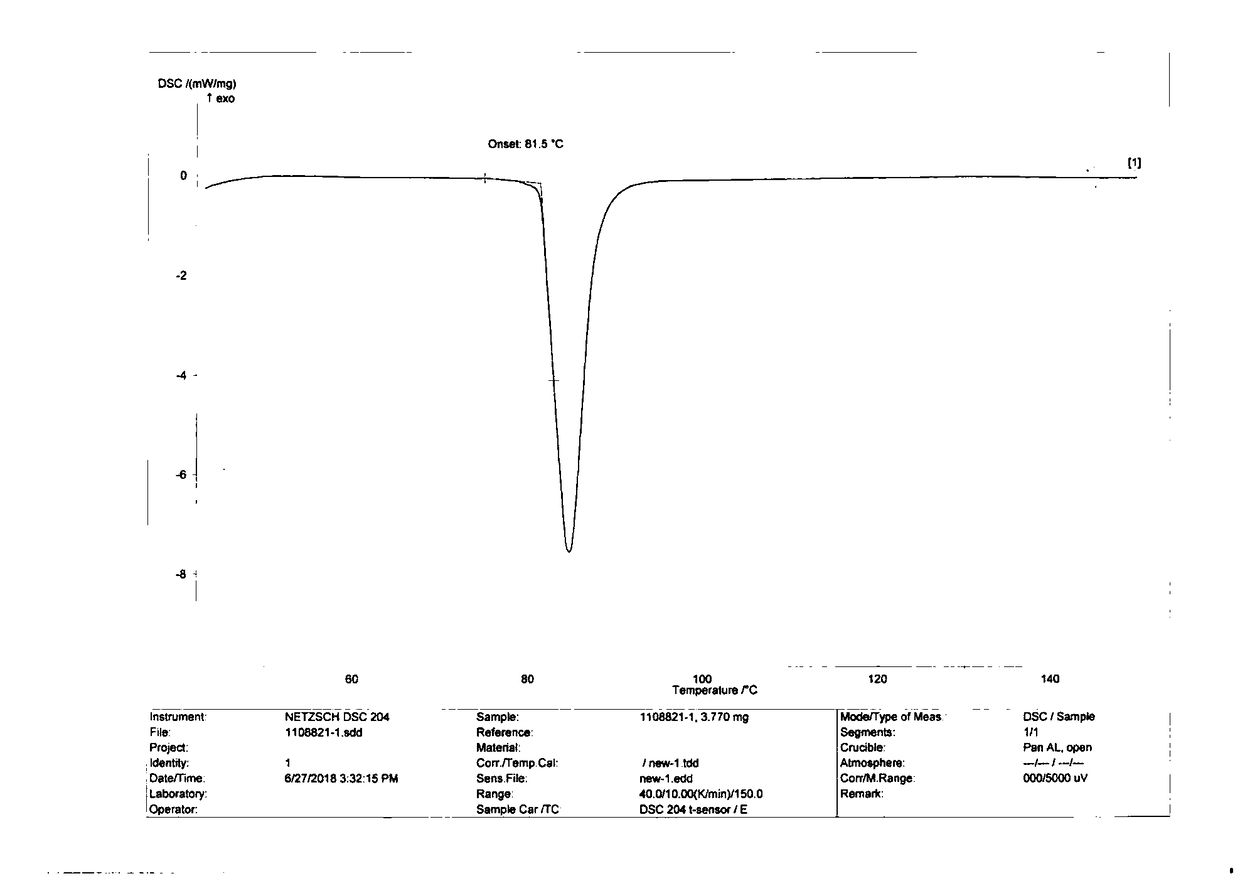
[0056] Gained crystal carries out X-ray powder diffraction (XRPD) analysis (see appendix figure 1 ), DSC analysis (see attached figure 2 ) and TGA analysis (attached image 3 ). The results indicated Form A.
Embodiment 2
[0058] Add 100g of vanillin, 500mL of water, and 100mL of ethanol into the reaction flask in sequence, raise the temperature to 75-85°C until clarified, remove insoluble matter by suction filtration while it is hot; heat the filtrate again until clarified, cool down to 10±5°C to crystallize for 6-8 Hour. Suction filtration, wash the filter cake (50mL×2). The filter cake was vacuum dried at 60°C to constant weight. 80.5 g of crystal form A was obtained, the HPLC purity was 99.92%, and the yield was 80.5%.
Embodiment 3
[0060]Add 100g of vanillin, 700mL of water, and 70mL of acetone into the reaction flask in sequence, raise the temperature to 60-70°C until clarified, remove insoluble matter by suction filtration while it is hot; heat the filtrate again until clarified, cool down to 10±5°C to crystallize for 6-8 Hour. Suction filtration, wash the filter cake (50mL×2). The filter cake was vacuum dried at 60°C to constant weight. 88.9 g of crystal form A was obtained, the HPLC purity was 99.96%, and the yield was 88.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com