Dioxygenase Snpd with chiral selectivity to intermediate of aromatic oxyphenoxypropionic acid herbicide, and encoding gene and application thereof
A dioxygenase, stereoselective technology, applied in the field of bioengineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
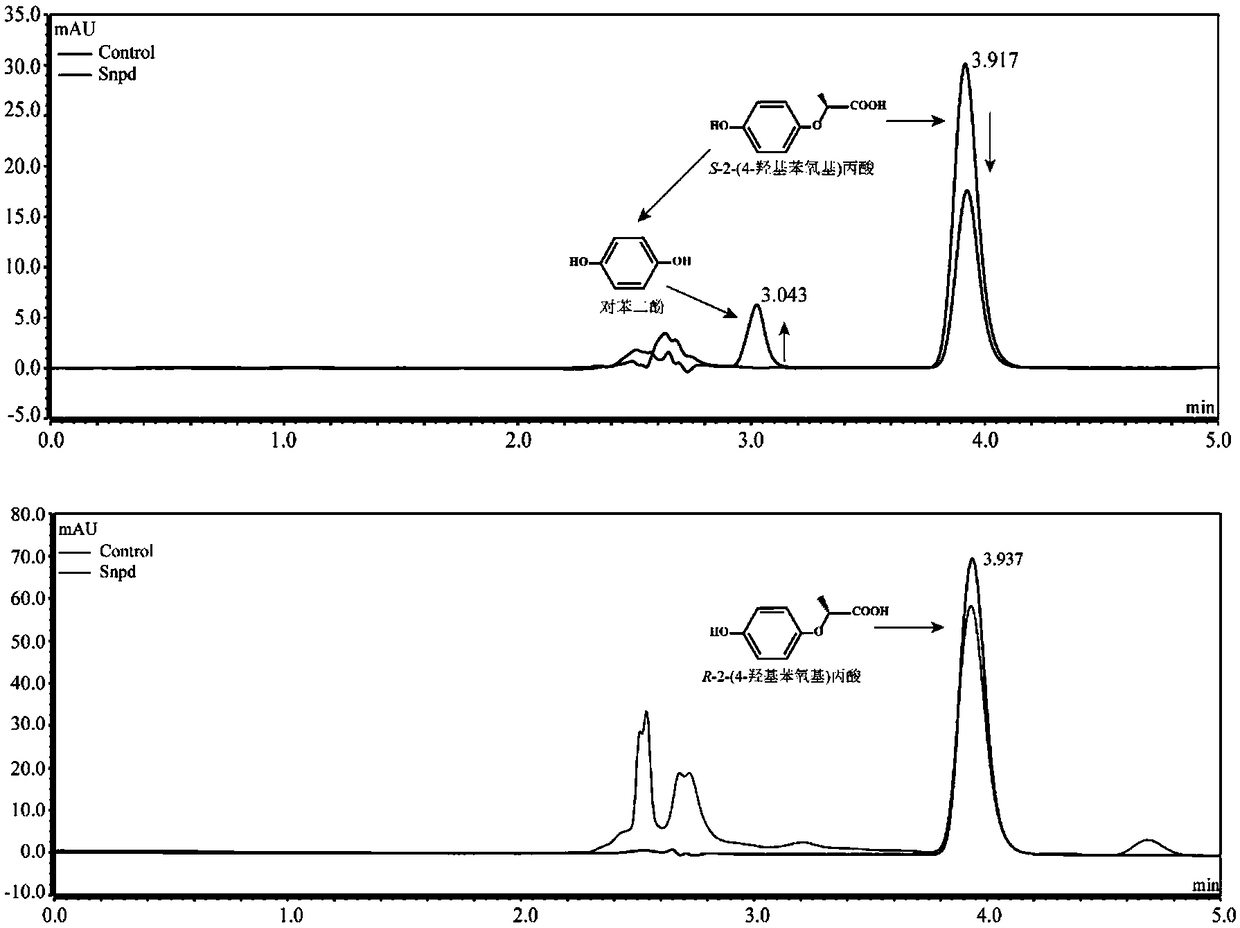
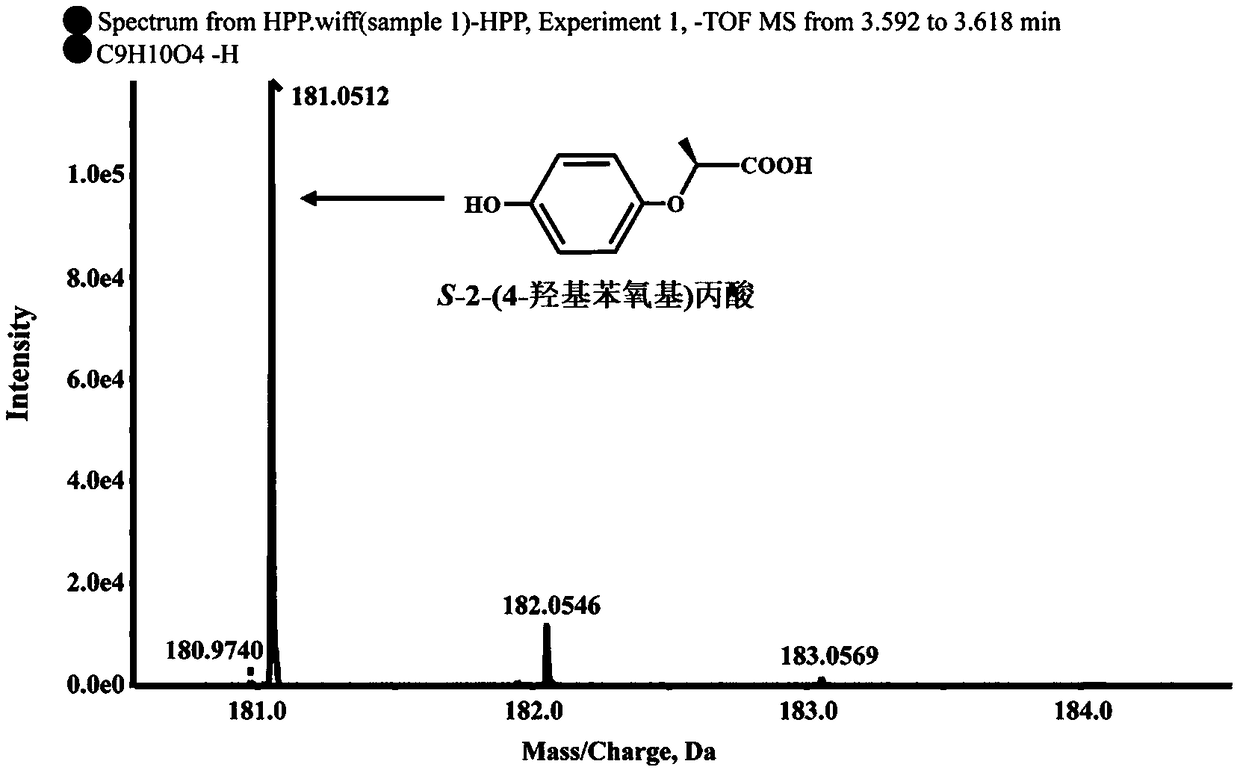
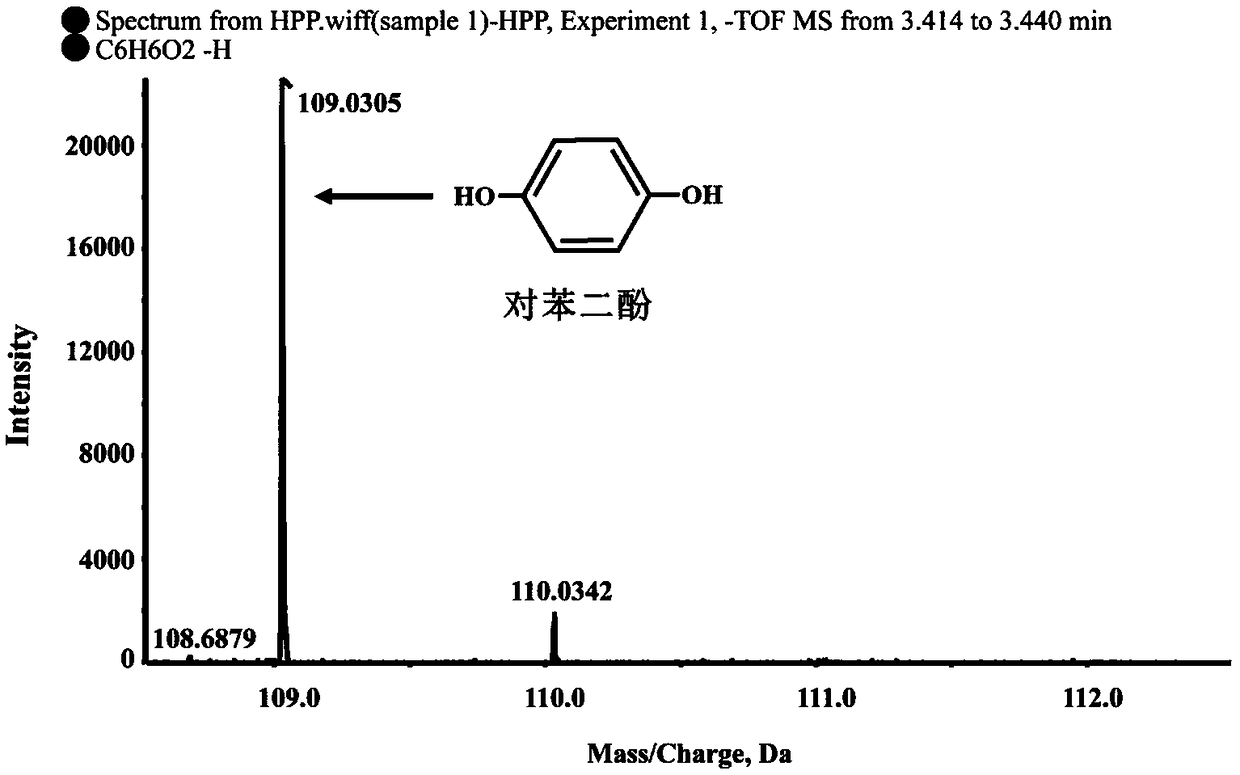
[0024] Determination of Degradation S-2-(4-hydroxyphenoxy)propionic acid metabolites by strain B2 of Example 1
[0025] Determination of degradation products: Inoculate strain B2 into 100mL LB liquid medium, culture at 30°C, 180rpm / min until exponential phase, collect bacteria by centrifugation at 6000rpm / min for 10min, wash the bacteria twice with sterile basic salt medium , resuspended in 10ml basal salt medium, inoculated into 100mL inorganic salt liquid medium, and adjusted OD 600 About 1.0, add 0.2mM S-2-(4-hydroxyphenoxy) propionic acid, shake culture at 30°C, 180rpm / min on a shaker. Samples were taken regularly every 3 hours, and an equal volume of methanol was added to the regularly sampled culture solution. After shaking and mixing, the samples were centrifuged at 12000 rpm / min for 5 minutes. The samples were filtered with a 0.22 μm nylon filter membrane and detected by high performance liquid chromatography (HPLC). The HPLC chromatographic conditions are: the chroma...
Embodiment 2
[0030] Example 2 Cloning and functional verification of S-2-(4-hydroxyphenoxy) propionic acid degradation gene
[0031] 2.1 Alignment and search of dioxygenase genes
[0032] Find out the dioxygenase gene tfdA that has been reported to catalyze similar reactions by consulting relevant literature. The TfdA protein sequence was compared with the genome of strain B2, and the result was that 3 similar protein sequences were compared in the genome of strain B2.
[0033] 2.2 Heterologous expression and functional verification of putative dioxygenase genes
[0034] Using the genomic DNA of strain B2 as a template, primers were designed to amplify the putative dioxygenase gene fragment. The primers used are as follows:
[0035] Table 1 Primers used in functional verification experiments
[0036]
[0037]
[0038] Amplification system:
[0039]
[0040] Amplification procedure
[0041] a. Pre-denaturation at 95°C for 3 minutes;
[0042] b. Denaturation at 95°C for 15 se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com