Pericyclic monosubstituted amphiphilic phthalocyanine photosensitizer and its preparation and application
A single-substituted, substituted group technology, applied in the field of photodynamic drug or photosensitizer preparation, can solve the problems of poor biological selectivity, unstable composition, high skin phototoxicity, etc., and achieves a reasonable and feasible preparation route and molar absorption coefficient. The effect of large, easily available synthetic raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
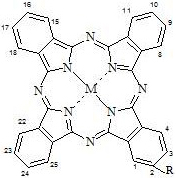
[0040] Synthesis and Physicochemical Properties of 1-(4-Sodium Sulfonate-Phenoxy) Phthalocyanine Zinc
[0041] ,
[0042] Formula 1)
[0043] This compound can also be called α-(4-sodium sulfonate-phenoxy)zinc phthalocyanine, and its structure is shown in formula (1), wherein:
[0044] .
[0045] (1) Preparation of 3-(4-sulfonic acid-phenoxy) phthalonitrile sodium salt: 3-nitrophthalonitrile (5mmol) and 4-hydroxybenzenesulfonate sodium (5~7.5mmol, Preferably 5mmol) as the reactant, dimethyl sulfoxide (10~25mL, preferably 10mL) as the solvent, in the presence of potassium carbonate (7.5~15mmol, preferably 10mmol, added in three batches) and nitrogen protection, room temperature~45°C Stir the reaction at 45°C (preferably 45°C) for 48-96 hours, monitor by thin-layer chromatography, and terminate the reaction when 3-nitrophthalonitrile is basically consumed. Filter the reaction mixture with a microporous organic membrane, collect the filtrate, then add the filtrate to 100m...
Embodiment 2
[0050] Synthesis and Physicochemical Properties of 1-(6,8-Potassium Disulfonate-2-Naphthyloxy) Phthalocyanine Zinc
[0051] ,
[0052] Formula 1)
[0053] This compound can also be called α-(6,8-disulfonic acid potassium-2-naphthyloxy)zinc phthalocyanine, and its structure is shown in formula (1), wherein:
[0054] .
[0055](1) Preparation of 3-(6,8-disulfonic acid-2-naphthyloxy) phthalonitrile dipotassium salt: with 3-nitrophthalonitrile (5 mmol) and 2-naphthol-6, Dipotassium 8-disulfonate (5~7.5mmol, preferably 5mmol) was used as reactant, dimethyl sulfoxide (10~25mL, preferably 10mL) was used as solvent, in potassium carbonate (7.5~15mmol, preferably 10mmol), divided into three In the presence of batch addition) and nitrogen protection, the reaction was stirred at room temperature to 45°C (preferably 45°C) for 48 to 96 hours, monitored by thin-layer chromatography, and the reaction was terminated when 3-nitrophthalonitrile was basically consumed. Filter the reactio...
Embodiment 3
[0060] Synthesis and Physicochemical Properties of 2-(4-Sodium Sulfonate-Phenoxy) Phthalocyanine Zinc
[0061] Replace the 3-nitrophthalonitrile in Example 1 with equimolar 4-nitrophthalonitrile to obtain the corresponding peripheral monosubstituted phthalocyanine metal complex, i.e. β-(4-sulfonic acid Sodium-phenoxy)zinc phthalocyanine. The structure of the obtained product is the same as that of the phthalocyanine product described in Example 1, except that the position of the substituent is replaced by the β position.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com